Treatment of Clinical Mastitis
Published: June 7, 2013
By: Pamela L. Ruegg, DVM, MPVM (University of Wisconsin, Madison)
Introduction
Mastitis continues to be the most frequent and costly disease of dairy cattle. Financial losses due to mastitis occur for both subclinical and clinical stages of the disease. Losses caused by subclinical mastitis are well documented. Each doubling of SCC above 50,000 cells/ml results in a loss of 0.4 kg and 0.6 kg of milk per day in first lactation and older cows, respectively (Hortet and Seegers, 1998). Losses caused by clinical mastitis include discarded milk, transient reductions in milk yield and premature culling (Fetrow, 2000). Perceived financial losses for clinical mastitis vary widely. In 2002-2003, farmers participating in milk quality programs in Wisconsin (n = 117) estimated that each clinical case of mastitis cost approximately $97.00 USD, but estimates ranged from zero to $260 per case. Discarded milk accounted for more than half of the cost ($54.00 USD).
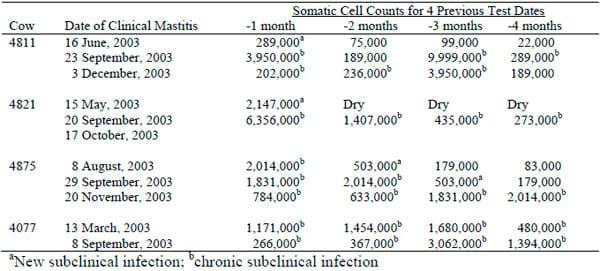
The frequency of new infections and the duration of existing infections determine the amount of mastitis in a herd (Ruegg, 2003). Mastitis should be considered a disease syndrome that often fluctuates between clinical and subclinical states (Table 1). Diagnostic methods for mastitis are not very sensitive and the reversion of a clinical case back to a subclinical state is often mistakenly considered as a cure.
Table 1. Somatic Cell Counts of Cows with Recurrent Cases of Clinical Mastitis
Prevention is the most cost-effective way to control mastitis, but effective treatment is necessary to produce high quality milk. Treatment is compulsory when a cow is obviously sick but in many instances, treatment of mastitis is voluntary. Treatment of mild or moderate cases of clinical mastitis should be considered when the probability of cure is high, the rate of recurrence is expected to decrease after treatment and a financial benefit to the farm is expected.
Principles of Treatment of Clinical Mastitis
The principles of successful treatment of clinical mastitis include the following:
1. Early detection of mastitis. The examination of foremilk before attaching the milking unit is extremely important to detect mild and moderate cases of mastitis. Long periods of subclinical mastitis infections allow some mastitis pathogens the opportunity to invade secretory tissue. In these cases, it is difficult for antibiotics to penetrate scar tissue and successfully destroy the bacteria. We monitored the number of days that milk was discarded for 225 cases of mild and moderate mastitis on a single dairy herd. Cows that had high somatic cell counts preceding the clinical case required longer treatment and had 9.5 days of milk discard as compared to 7.0 days discarded for cows that had low SCC before developing clinical mastitis (P = 0.006).
2. A presumptive diagnosis of the pathogen. Knowledge of the causative pathogen is required for appropriate treatment. Intramammary antibiotics are not effective when given to cows with mastitis caused by some pathogens (for example, Mycoplasma spp, yeast, chronic S aureus infections and mild infections caused by Gram-negative pathogens). Other pathogens, such as Strep uberis and newly acquired Staph aureus infections, may require longer duration of intramammary antibiotic therapy to ensure that the infection is fully cured.
3. Knowledge of the probability of successful treatment. Cure rates for treatment vary depending on the pathogen, duration of infection and characteristics of the cow. It is not cost effective to repeatedly use intramammary antibiotics to treat chronic S aureus infections in cows that are unlikely to respond. It is however, important to use intramammary antibiotics for appropriate periods to treat cows with mastitis caused by Streptococcus spp.
Mastitis Pathogens & Clinical Mastitis
Clinical mastitis can be caused by virtually any mastitis pathogen. A presumptive diagnosis of the pathogen responsible for clinical mastitis is important because the probability of successful treatment is related to characteristics of the pathogens. In recent years the proportion of mastitis caused by Strep agalactiae and Staph aureus has decreased (Makovec and Ruegg, 2003). In many herds, Gram-negative bacteria are responsible for a large proportion of clinical mastitis. We recently collected quarter milk samples from mastitic cows on 5 commercial dairy farms that had very little contagious mastitis. Milk samples were collected only from cows that did not have systemic signs of disease (such as fever, anorexia or pain). Coliform bacteria were isolated from 58 (44.3%) of the cases (n = 131), while environmental Streptococci (33.6%), coagulasenegative Staphylococci (18.3%) and a variety of other bacteria (3.8%) were responsible for the remaining cases.
In a separate study, we cultured cases (n = 241) of mild and moderate clinical mastitis that had been identified by milking technicians on a single Wisconsin dairy farm. Coliform bacteria (34.4%) and bacteriologically negative samples (34.4%) were the most common results. Other isolates included: Staph aureus (5.8%), environmental Streptococci (6.2%), coagulase negative Staphylococci (8.7%), Mycoplasma bovis (3.7%) and others (6.6%).
Treatment of Clinical Mastitis Caused by Specific Pathogens
Streptococcus agalactiae: Strep ag lives only in the udder of cows and is not a frequent cause of clinical mastitis in most herds. Intramammary treatment with penicillin type drugs continues to be highly effective resulting in 80-90% cure rates. To eradicate Strep ag, all 4 quarters of all culture positive cows in the herd should be treated with an appropriate commercially marketed intramammary antibiotic (Erskine, 2001). A small percentage of animals will not be cured, therefore cows that continue to have high SCC values should be resampled and cultured at 30-day intervals. Cows that remain infected can be retreated but should be segregated from the herd to prevent reinfection. Treatment of the herd should be accompanied by an effective teat dipping program and comprehensive dry cow therapy. Treatment of cows subclinically infected with Strep ag usually results in increased production and dramatic decreases in bulk tank SCC values.
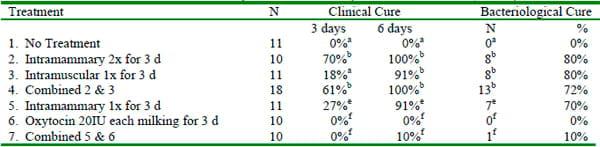
Environmental Streptococci: The spontaneous cure rate for clinical mastitis caused by environmental Streps (usually Strep uberis and Strep dysgalactiae) may exceed 50%, but frequent relapses occur if the cows do not receive appropriate antibiotic therapy (Morin et al, 1998). Clinical cases of mastitis caused by environmental streps should be treated with approved intramammary antibiotics for an appropriate number of treatments. The use of aggressive treatment of induced Strep uberis infections has been shown to result in cure rates that exceed 90% (Table 3; Hillerton and Kleim, 2002). The use of oxytocin as an adjunct therapy was not effective in this study.
Table 3. Cure rates for induced Strep uberis mastits (Hillerton & Kleim, 2002).
Staph aureus: There are a number of factors that influence the cure rate for cows infected with Staph aureus (Owens et al., 1997; Sol et al., 1997). One study reported that bacteriologic cure rates for newly acquired (< 2-weeks duration) Staph aureus infections were 70% (Owens, et al., 1997). The study used intramammary treatment with a commercially available penicillin novobiocin product. Cure rates for chronic (> 4- weeks duration) Staph aureus infections were only 35%. Cure rates for mastitis caused by Staph aureus have been shown to decrease with age (from 81 % for cows <48 months of age to 55% for cows >96 months), the number of infected quarters (from 73% for 1 infected quarter to 56% for 4 infected quarters) and SCC (Sol et al., 1997). Cows infected in more than 1 quarter were less than half as likely to be cured as compared to cows with only 1-quarter infected (Sol et al., 1997). In general, treatment of cows infected with Staph aureus may be successful when infections are of short duration (< 2- weeks), in young cows and in early lactation. The use of extended duration of intramammary therapy (8 days) may further improve cure rates (Deluyker et al., 2001, Ruegg and Araujo, 2002).
Coagulase-negative Staphylococci: CNS are frequently isolated from milk samples in herds that have controlled major pathogens (Harmon et al, 1995). While CNS are not a frequent cause of clinical mastitis, surveys in herds that have controlled major pathogens generally attribute 3-10% of clinical cases to CNS. CNS live on teat skin and can colonize the teat canal. Dry cow therapy is usually effective in controlling these organisms. The rate of spontaneous cure is high but intramammary treatment of cows infected with CNS is often highly successful (Wilson et al., 1999).
Gram-negative bacteria: The use of J-5 vaccines has reduced the amount of severe mastitis caused by Gram-negative bacteria. Most mastitis caused by Gram-negative bacteria is mild or moderate because the immune response is highly successful in destroying these bacteria. As the bacteria are destroyed, they release endotoxin from their cell walls. In 5-15% of these cases, enough endotoxin is released to result in seriously ill cows. These cows require rapid diagnosis and immediate supportive therapy. The hydration status of the cows should be evaluated and cows should be given hypertonic or isotonic fluid therapy and appropriate anti-inflammatories. In more than 40% of severely ill animals, bacteria may escape the udder and circulate throughout the bloodstream (Wenz, et al., 2001). A recent study demonstrated more favorable clinical outcomes for cows with severe clinical coliform mastitis that received IM ceftiofur once daily as compared to cows that received only supportive therapy (Erskine et al., 2002).
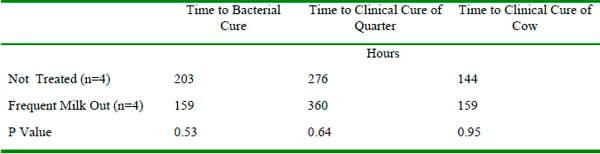
The use of oxytocin and frequent milking is often recommended as adjunct therapy for subacute and acute coliform mastitis. Improvements in clinical outcomes have not been reported in the two small studies that have evaluated these strategies (Leininger et al., 2003, Roberson, 1997)(Table 4).
Table 4. Outcomes of experimentally induced E. coli mastitis (Leininger et al. 2003)
Selection of Antibiotics
Many strains of contagious mastitis pathogens establish subclinical infections that persist in the udder for long periods of time without detection. These pathogens may cause periodic episodes of mild to moderate mastitis that seem to resolve without treatment. In most instances, the cow remains subclinically infected and the infections are simply alternating between subclinical and clinical states. Bacteria are often shed intermittently and antibiotics (during lactation or at dry off) are required for treatment. These organisms are usually gram positive and in many cases intramammary mastitis tubes may be recommended for effective treatment.
In contrast, opportunistic bacteria that reside in the environment of the cows tend to be less adapted to survival in the udder and often stimulate an acute immune response when they infect the udder. The immune response is usually successful in eliminating these pathogens resulting in a high rate of spontaneous cure. Consequently, the natural duration of infection is often relatively short and the only sign of infection may be a brief period of abnormal milk with or without changes in appearance of the udder. These organisms are usually gram negative and intramammary mastitis tubes are often not required.
The use of susceptibility testing to guide treatment decisions is common. In recent years, antimicrobial susceptibility testing has come under scrutiny because of concerns about antimicrobial resistance, changes in methodology and the relationship between in-vitro results and on-farm clinical outcomes. Susceptibility tests of milk samples submitted to state diagnostic laboratories that use the disk-diffusion method have demonstrated remarkable agreement but vary from results of a small survey processed using broth dilution (Table 5).
Table 5. Proportion of isolates susceptible
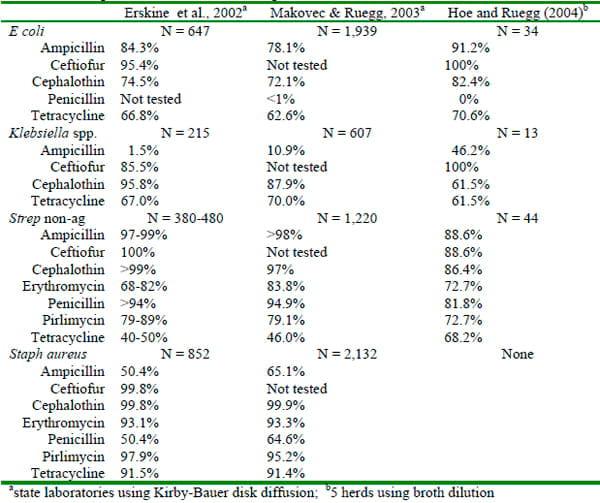
It is interesting to note the large proportion of Staph aureus isolates are susceptible invitro, yet the clinical experience with treatment of Staph aureus is often frustrating. The relationship between in-vitro susceptibility test results and clinical outcomes has not been well documented (Constable and Morin, 2003). Several studies have demonstrated little relationship between susceptibility results and bacteriological cure rates, while others have demonstrated higher cure rates for susceptible bacteria when the causative pathogen was gram-positive. Preliminary results of a study comparing bacteriological and clinical outcomes of resistant and susceptible bacteria (determined using broth dilution) isolated from mild and moderate cases of clinical mastitis are shown in Table 6. Bacteriological cure rates were low but a rigorous definition of cure was used (negative duplicate milk samples).
Table 6. Preliminary results of clinical mastitis outcomes (Hoe and Ruegg, 2004).
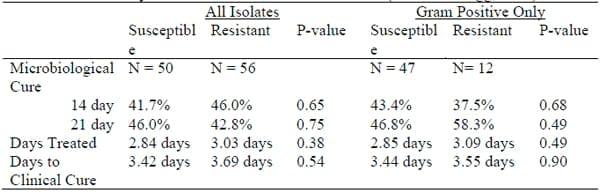
The lack of pharmacodynamic data for intramammary antibiotics and lactating dairy cows is probably responsible for the inability to relate clinical outcomes of mastitis therapy to susceptibility tests. At this point, more research is needed to determine the validity of susceptibility tests and how to apply the in-vitro results to clinical cases. Until more data is obtained, selection of antibiotics should be based on knowledge of the underlying pathogens and clinical trials that have established successful therapeutic protocols.
Using On-Farm Treatment Protocols for Clinical Mastitis
It is extremely important to have a treatment plan for clinical mastitis that is based upon the individual herd history and a likely diagnosis of the suspected pathogen. The diagnostic plan should include routine culturing of at least some cases of clinical mastitis. Even when milk samples are routinely collected from cases of clinical mastitis, treatment of clinical mastitis will usually occur before the diagnosis is known. A history of previous diagnosis and a careful physical exam should be used to guide treatment decisions. With the exception of chronic infections caused by Staph aureus, intramammary antibiotic therapy should be used to treat cases that are suspected to caused by Gram-positive bacteria. The use of intramammary and systemic antibiotics to treat mastitis that is probably caused by Gram-negative bacteria should be reserved for seriously ill cows. Antibiotics should be given for appropriate periods of time. Aggressive treatment with intramammary tubes may include extended time periods or tubes given at each milking rather than once per day (Hillerton and Kliem, 2003). Responses to therapy should be monitored using practical values such as the number of days milk is withheld from sale and relapse rates after treatment.
The use of treatment protocols based on culture results has been described (Hess, et al., 2003). We recently modified this procedure to use a commercial culture system to guide mastitis therapy on a large dairy farm (Figure 1). Farm personnel performed the cultures and read them after 24 hours of incubation.
Figure1. Treatment protocol using on-farm culturing to direct therapy
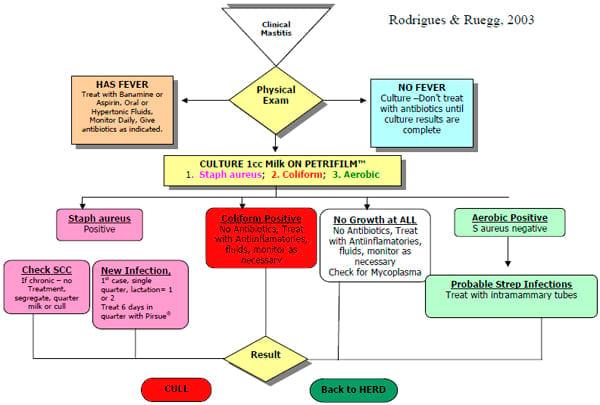
We compared the clinical outcomes of 240 cases of clinical mastitis that were treated according to this protocol to outcomes of 100 cases of clinical mastitis that occurred immediately before the protocol was adopted. In the pretrial period, milk was discarded for an average of 20 days for each mastitis case as compared to milk discarded for 8 days during the trial. Only 13 of 100 (13%) of the pretrial cases did not receive intramammary antibiotics as compared to 161 (67%) cases treated during the protocol period. In this herd, the use of a standardized treatment protocol and directed antibiotic therapy resulted in less antibiotic usage and more favorable clinical outcomes.
Conclusion
Treatment is an important aspect of mastitis control. The most effective treatment strategies include early detection, presumed identification of mastitis pathogens and the use of antibiotics for an appropriate duration for the expected pathogen. The use of onfarm treatment protocols can dramatically improve outcomes of clinical mastitis treatment.
References
Constable, P.D., and D. E. Morin. 2003. Treatment of clinical mastitis using antimicrobial susceptibility profiles for treatment decisions. in Veterinary Clinics of North America. Food Animal Practice. 19(1):139-155.
Deluyker, H. A., P. Michanek, N. Wuyts, et al., 2001. We treat sick cows don't we? The case of subclinical mastitis. pp 170-174, in Proceedings of the 40th annual meeting of Natl. Mast. Coun., Reno NV, Natl Mast Coun. Madison, WI.
Erskine RJ. 2001. Mastitis Control in Dairy Herds. Chap 10 in Herd Health: food animal production medicine. 3rd ed. Radostits editor. WB Saunders, Philadelphia.
Erskine R.J, P.C. Bartlett, P. C. Crawshaw, and D.M. Gombas. 1994. Efficacy of intramuscular oxytetracycline as a dry cow treatment for Staphylococcus aureus mastitis. J Dairy Sci 77:3347-3353.
Erskine R. J., P.C. Bartlett, J. Van Lente, et al., 2002. Efficacy of systemic ceftiofur as a therapy for severe clinical mastitis in dairy cattle. J Dairy Sci 85:2571-2575.
Fetrow, J. 2000. Mastitis: an economic consideration. pp 3-47 in Proceedings of the 29th annual meeting of Natl. Mast. Coun., Atlanta, GA, Natl Mast Coun. Madison, WI.
Harmon, R. J., and B. E. Langlois. 1995. Mastitis due to coagulase-negative Staphylococcus species. Pp 56-64, in Proc Natl Mastitis Counc. Vol 34, National Mastitis Council, Madison WI.
Hess, J.L., L. M. Neuder and P. M. Sears. 2003. Rethinking clinical mastitis therapy. Pp 372-373 in Proc Natl Mastitis Counc. Vol 42, National Mastitis Council, Madison WI.
Hillerton, J. E. and K. E. Kliem. 2002. Effective treatment of Streptococcus uberis clinical mastitis to minimize the use of antibiotics. J Dairy Sci 85:1009-1014.
Hortet P, H. Seegers. 1998. Calculated milk production losses associated with elevated somatic cell counts in dairy cows: review and critical discussion. Vet Res. 29(6):497-510.
Leininger, D. J., J. R. Roberson, F. Elvinger, D. Ward, and M. Akers. 2003. Evaluation of frequent milkout for treatment of cows with experimentally induced Escherichia coli mastitis. J Am Vet Med Assoc. 222:63-66.
Makovec, J. A., and P.L. Ruegg. 2003. Characteristics of milk samples submitted for microbiological examination in Wisconsin from 1994 to 2001. J Dairy Sci 86:3466-3472.
Morin D.E., R. D. Shanks, and G.C. McCoy. 1998. Comparison of antibiotic administration in conjunction with supportive measures versus supportive measures alone for treatment of dairy cows with clinical mastitis. J Am Vet Med Assoc. 213:676-684.
Owens WE, Ray CH, Watts JL, Yancey RJ. 1997. Comparison of success of antibiotic therapy during lactation and results of antimicrobial susceptibility tests for bovine mastitis. J Dairy Sci 80:313- 317.
Roberson, J. R. Frequent milk-out as a treatment for subacute clinical mastitis. Pp 152-157in Proc Natl Mastitis Counc. Vol 36, National Mastitis Council, Madison WI.
Ruegg, P.L. 2003. Investigation of Mastitis problems on farms. in Veterinary Clinics of North America. Food Animal Practice. 19(1):47-74.
Ruegg, P.L, and T. P. B., Araujo, 2002. Effect of extended therapy of subclinical mastitis pathogens. 2nd Panamerican Congress on Milk Quality and Mastitis Control. Nov 25-27, 2002. Ribeirao Preto, Brazil.
Sol J, O. C. Sampimon, J. J. Snoep and Y. H. Schukken. 1997. Factors associated with bacteriological cure during lactation after therapy for subclinical mastitis caused by Staphylococcus aureus J Dairy Sci 80:2803-2808.
Wenz, J. R., G. M. Barrington, F. B., Garry, et al., 2001. Bacteremia associated with naturally occurring acute coliform mastitis in diary cows. J Am Vet Med Assoc 219:976-981.
Wilson D.J., R. N. Gonzalez, K. L. Case, L. L. Garrison, and Y. T. Grohn. 1999. Comparison of seven antibiotic treatments with no treatment for bacteriological efficacy against bovine mastitis pathogens. J Dairy Sci 82:1664-1670.
This article was originally published on the Milk Quality, University of Wisconsin´s website. http://milkquality.wisc.edu/. Engormix.com thanks for this huge contribution.
Related topics:
Authors:

Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.








