Factors Affecting Fish Blood Profile: C- Effect of Other Environmental and Genetic Factors
Published: February 22, 2021
By: Abdelhamid M. Abdelhamid 1; Mohamed M. A. H. Refaey 1; Mahmoud F. Salem 2 and Mostafa A. M. El-Kattan 1./ 1 Animal Production Department, Faculty of Agriculture, Mansoura University, Egypt; 2 Aquaculture Research Unit, Sakha, Central Lab. of Aquaculture Research, Agricultural Research Center, Cairo, Egypt.
Summary
Many studies in the fields of fish nutrition, reproduction, and biology including blood tests revealing factors influence blood composition. In the present part of the serial studies, more lights are given on effects of fish species, sex and size as well as different water bodies' locations (lake, farm, Nile) and water salinity and temperature on fish blood changes.
Introduction
Penzlin (1977) cited that blood constituents of fish are variable than those of the terrestrial animals. Abdelhamid (1991) argumenta for the variation between fish and terrestrial animals to the huge number of fish species (ca. 25.000) and strains (40.000) in comparison with the mammalian species (ca. 4.500). Additionally, Abdelhamid (2009) cited that fish are variable in their morphology, biology, anatomy, physiology, reproduction, respiration, body composition, and blood profile. Blood parameters of fish are useful tools that aid in the immnuopotentiators studies (Ellis, 1981). Press and Evensen (1999) expected species variation in the morphology of the immune system within the teleost fishes. However, data available about the ontogenic development of the innate immunological system in fish is limited (Magnadóttir, 2006). Recently, some factors were studied as affecting fish blood profile (Abdelhamid et al., 2019a & b). The present study was designed to evaluate the effect of some other factors affecting some blood parameters. These factors included fish species and size, water salinity, and rearing conditions; since, it was given in Verse 12 of Sura Faatir of Holly Quran concerning the variations in water and fish composition: "And not alike are the two bodies of water. One is fresh and sweet, palatable for drinking, and one is salty and bitter. And from each you eat tender meat and extract ornaments which you wear, and you see the ships plowing through [them] that you might seek of His bounty; and perhaps you will be grateful". So, this was also included in the objectives of the present study.
Materials and mothods
Random fish samples were taken for blood collected from the caudal peduncle by special syringe. Five-ml-syringes were used for blood withdrawal into two types of test tubes, the 1st with ethylene diamine tetra acetic acid (EDTA) for the haematological parameters and the 2nd without EDTA for the biochemical parameters. Blood plasma was obtained by centrifugation at 3500 rpm for 15 min. Dragoon pipets were used for withdrawing the samples from the test tubes as shown from the following pictures. Blood plasma samples were used for determination of creatinine (Tietz, 1986), triglycerides (McGowan et al., 1983), total proteins (Tietz, 1990) and albumin (Wotton and Freeman, 1982) concentrations as well as the activity of aspartate amino transferase (AST) and alanine amino transferase (ALT) using commercial test kits (Humalyzer 3000 manufactured by Human, Germany) in a private lab. in Kafr El-Sheikh governorate, Egypt. Globulin level was calculated by subtracting albumin from total protein. The other samples of blood were used to determine the blood hematology as concentration of hemoglobin (Hb), total count of erythrocytes (RBCs), and total leukocytes (WBCS) (Natt and Herrick, 1952) and hematocrit (Hct) using Auto Counter (920 EO+ manufactured by Swelab Switzerland) (Decie and Lewis, 2006) in the same lab. The other hematological parameters were mathematically calculated. Blood analysis data was statistically analyzed according to SAS (2006). When F- test was significant at P≤0.05, Duncan's test (1955) was carried out.
Results and discussion
- Five fish (250±3 g for each sex, species, and location) from each fish species (Nile tilapia, Oreochromis niloticus and African catfish, Clarias gariepinus), fish sex (male and female), and location (Lake Borulus, a fish farm, and the River Nile, Rosetta branch) were chosen for blood collection and analysis during September 2018. The water quality in these locations were as follow:
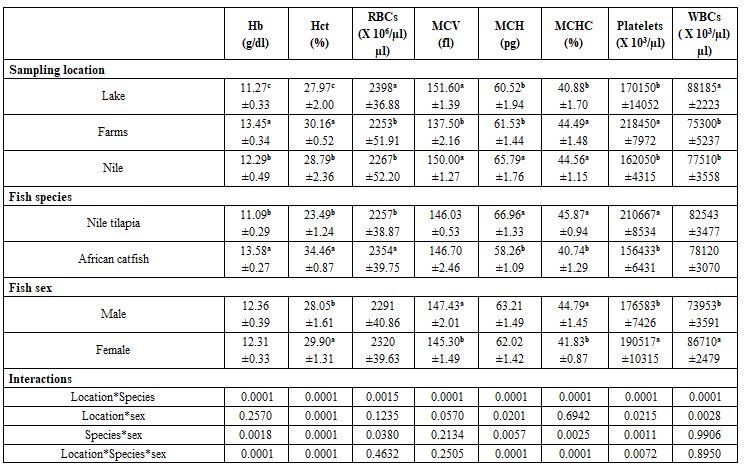
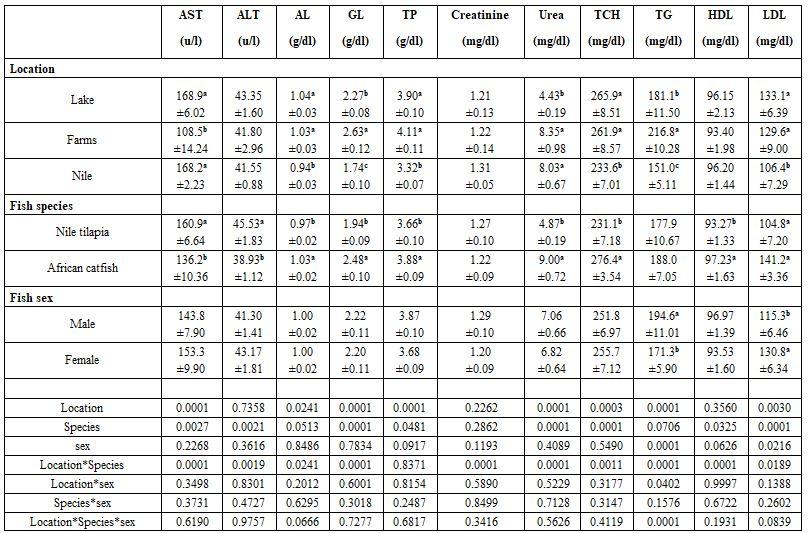
Tables 1 and 2 show that sampling location significantly (P≤0.05) affected all haematological and most biochemical parameters measured. That may be attributed to the wide variations in their water conditions, particularly to their salinity (1.1-5.3 g/l), ammonia (0.64-1.2 mg/l), and dissolved oxygen (4.3-7.8 mg/l). The same trend was recorded for fish species, it significantly (P≤0.05) affected also all haematological and most biochemical parameters under study. Fish sex was less affecting the blood profile, particularly the biochemical ones. Therefore, the interactions were more significant among the haematological than among the biochemical parameters. Abdel-Moneim et al. (2013) found that increasing ammonia in the rearing water of tilapia was responsible for decreased serum total iron, total binding iron capacity, amylase and lipase while insulin significantly increased. Also, Hassanen and Abd Elnabi (2017) found that increased ammonia level in red tilapia rearing water led to higher urea, uric acid and creatinine values in the fish blood. Also, Moreover, Abd El-Ghaffar and Abu El- Nasr (2017) confirmed that rearing water pH affects significantly all blood physiological parameters of Nile tilapia. Helal et al. (2018) studied also the harmful effects of water pollution on some physiological responses of the Nile tilapia (Oreochromis niloticus) in both Qarun and Burullus Lakes These findings are in accordance with those found by Abdelhamid et al. (2019b) concerning the effect of farm location and condition, fish species, and water salinity.
Table 1: Effect of sampling locations and fish species and sex on some haematological parameters (means* ± standard errors)
n= 5, a-c: means superscripted with different letters significantly (P≤0.05) differ, Hb: haemoglobin, Hct: haematocrit, RBCs: red blood cells, MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin, MCHC: mean corpuscular haemoglobin concentration, WBCs: white blood cells.
Table 2: Effect of sampling locations and fish species and sex on some blood biochemical parameters (means* ± standard errors)
*: n= 5, a-c: means superscripted with different letters significantly (P≤0.05) differ, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GL: globulin, TP: total protein, TCH: total cholesterol, TG: triglycerides, HDL: high density lipoprotein, LDL: low density lipoprotein.
Water salinity is an important factor; since it was given in Verse 12 of Sura Faatir (No.35 of Holly Quran) concerning the variations in water comLocation: "And not alike are the two bodies of water. One is fresh and sweet, palatable for drinking, and one is salty and bitter. And from each you eat tender meat and extract ornaments which you wear, and you see the ships plowing through [them] that you might seek of His bounty; and perhaps you will be grateful". Also, in Surah Ar-Rahman (The Most Graciouse, No. 55 of Holly Quran) in Verses 19-21: " He released the two seas, meeting [side by side] (19); Between them is a barrier [so] neither of them transgresses (20); So which of the favors of your Lord would you deny? (21). For this reason, Nile fish [(5 of each Nile tilapia Oreochromis niluticus (150g) and Tilapia aurea, Trewavas, 1965 (160g)] and sea fish [(5 of each sea bass, 125g and Tobara mullet (Liza ramada), 140g)] were collected to examine their blood. The water conditions of Nile [inshore, in front of the Barzakh (Isthmus/Barrier that found between fresh and salt water)] and sea [off-shore, behind the Barzakh (Isthmus/Barrier) that found between fresh and salt water] in Rosetta Strait region (where the Barzakh (Isthmus/Barrier) is given in Holly Quran) were:
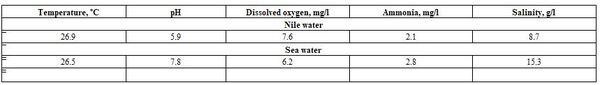
Tables 3 and 4 revealed significant (P≤0.05) variation between Nile (fresh water, 2 species, each 5 fishes) and sea (salt water, 2 species, each 5 fishes) in all haematological parameters, except platelets and white blood cells (WBCs) counts; yet most biochemical parameters did not influence, except globulin (GL), total protein (TP), total cholesterol (TCH), and high density lipoprotein (HDL). Sea fish (sea bass and Tobara) had often higher values than for Nile fish [Oreochromis niluticus and Tilapia aurea], except for MCHC. This may be due to the effect of water salinity (8.7 vs. 15.3 g/l) which affects not only fish blood characteristics but mainly fish species, which is suitable according to each water salinity; hence, fish species are classified into freshwater fishes and saltwater fishes (Abdelhamid, 2019a & b). As said before, the God in Holly Quran, since 1440 years ago (Hegira of profit Mohamed), stated about the variation between fresh and salt water.
Table 3: Effect of sampling locations from Rosetta Strait on some haematological parameters (means* ± standard errors)
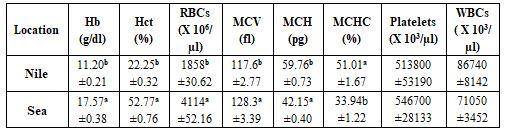
*: n= 10 (collective of 2 species, 5 each), a-b: means superscripted with different letters significantly (P≤0.05) differ, Hb: haemoglobin, Hct: haematocrit, RBCs: red blood cells, MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin, MCHC: mean corpuscular haemoglobin concentration, WBCs: white blood cells.
Table 4: Effect of sampling locations from Rosetta Strait on some blood biochemical parameters (means* ± standard errors)
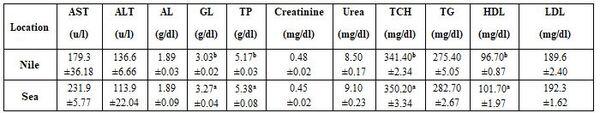
*: n= 10 (collective of 2 species, 5 each), a-b: means superscripted with different letters significantly (P≤0.05) differ, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GL: globulin, TP: total protein, TCH: total cholesterol, TG: triglycerides, HDL: high density lipoprotein, LDL: low density lipoprotein.
- Fish size (regardless to sampling location, sex, rearing condition…etc. as a cumulative sample) of Nile tilapia significantly (P≤0.05) Hb, MCH, MCHC, platelets, and most biochemical parameters, except urea and HDL (Tables 5 and 6). Effect of fish size on its blood profile was studied too by Abdelhamid et al. (2019a).
Table 5: Effect of Nile tilapia size on some haematological parameters (means* ± standard errors)
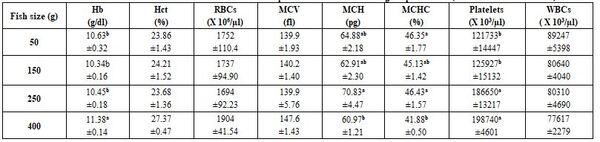
*: n= 30 (cumulative sample), a-c: means superscripted with different letters significantly (P≤0.05) differ, Hb: haemoglobin, Hct: haematocrit, RBCs: red blood cells, MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin, MCHC: mean corpuscular haemoglobin concentration, WBCs: white blood cells.
Table 6: Effect of Nile tilapia size on some blood biochemical parameters (means* ± standard errors)
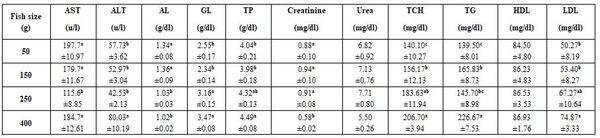
*: n= 30 (cumulative sample), a-c: means superscripted with different letters significantly (P≤0.05) differ, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GL: globulin, TP: total protein, TCH: total cholesterol, TG: triglycerides, HDL: high density lipoprotein, LDL: low density lipoprotein.
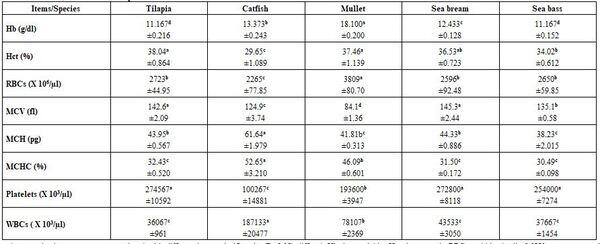
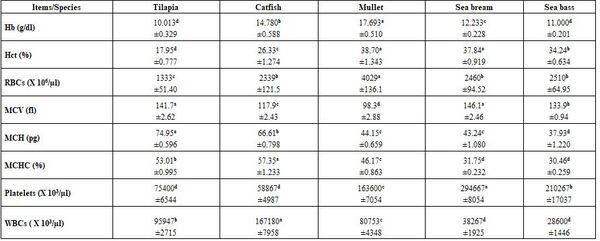
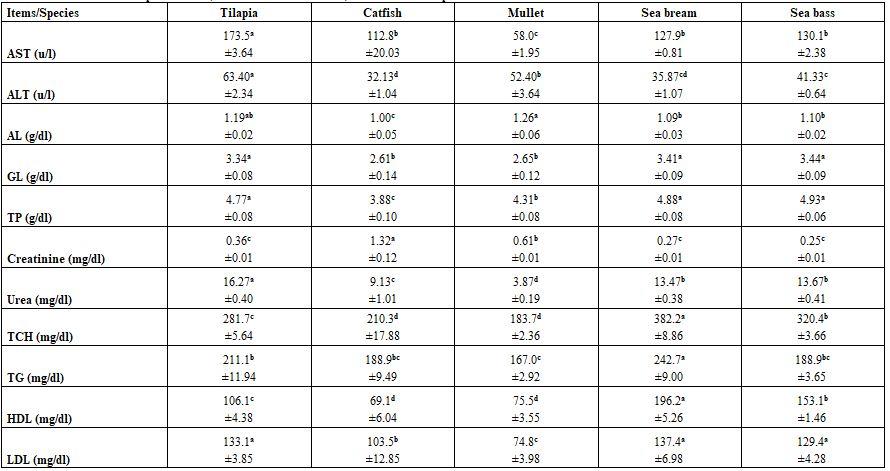
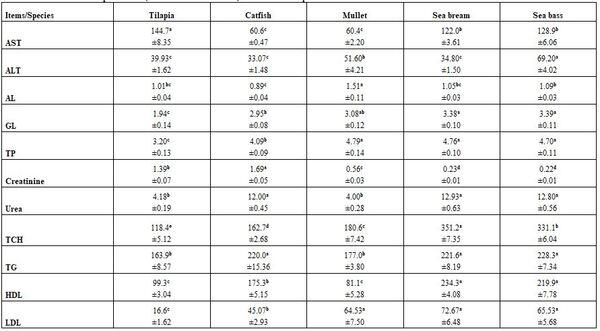
- Regardless to other variables, fish species and water temperature significantly (P≤0.05) affected all tested blood parameters, whether haematological or biochemical (Tables 7 - 10) without concerning trends for all species within all parameters at 30 as well as at 10°C. There was no specific effect of water temperature on different blood parameters tested.
Table 7: Haematological parameters (means* ± standard errors) of different fish species at 30°C
*: n= 15 (collective sample), collective sample, a-d: means in the same row superscripted with different letters significantly (P≤0.05) differed, Hb: haemoglobin, Hct: haematocrit, RBCs: red blood cells, MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin, MCHC: mean corpuscular haemoglobin concentration, WBCs: white blood cells.
Table 8: Haematological parameters (means* ± standard errors) of different fish species at 10°C
*: n= 15 (collective sample), collective sample, collective sample, a-d: means in the same row superscripted with different letters significantly (P≤0.05) differed, Hb: haemoglobin, Hct: haematocrit, RBCs: red blood cells, MCV: mean corpuscular volume, MCH: mean corpuscular haemoglobin, MCHC: mean corpuscular haemoglobin concentration, WBCs: white blood cells.
Table 9: Blood biochemical parameters (means* ± standard errors) of different fish species at 30°C
*: n = 15 (collective sample), a-d: means in the same row superscripted with different letters significantly (P≤0.05) differed, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GL: globulin, TP: total protein, TCH: total cholesterol, TG: triglycerides, HDL: high density lipoprotein, LDL: low density lipoprotein.
Table 10: Blood biochemical parameters (means* ± standard errors) of different fish species at 10°C
*: n= 15 (collective sample), a-d: means in the same row superscripted with different letters significantly (P≤0.05) differed, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GL: globulin, TP: total protein, TCH: total cholesterol, TG: triglycerides, HDL: high density lipoprotein, LDL: low density lipoprotein.
Press and Evensen (1999) mentioned that species variation in the morphology of the immune system is to be expected, given the large number and diversity of species within the teleost fishes. Dominguez et al. (2004) confirmed that elevating rearing water temperature (18.4, 23, and 28 ºC) for 2 weeks increased the circulating IgM concentration in Nile tilapia, but rearing the fish at 33 ºC resulted in a decrease in IgM concentration. These results suggest that the specific immune system of tilapia changes by certain factors in aquatic environment. Abdelhamid et al. (2015) revealed that catfish gave significantly higher values for most of the hematological parameters referring to best tolerance among the studied fish species. Except MCV, all other hematological parameters reflected significant interaction effects (diet X species), where the highest Hb and MCV values were of catfish fed the control diet, but sewage sludge fed catfish gave the highest MCHC and WBCs, catfish fed the sewage sludge had also the highest RBCs and PCV, and control fed tilapia only had the highest MCH value.
Conclusively: These results confirm that fish blood parameters are strong variable influenced by fish species, size, and sex as well as by the location, and rearing water temperature and salinity. Therefore, the interpretation of blood data of fish must be done on light of the specific individual experiment condition because of absence of referenced ranges for fish haematological and biochemical measurements.
References
- Abd El-Ghaffar, H.A. and Abu El- Nasr, T.M. (2017). Effects of pH on growth index, blood physiological parameters and some of digestive enzymes of the Nile tilapia fish Oreochromis niloticus (Linnaeus, 1758). 1st Inter. Conf. on "Sustainable Development of Aquaculture" The Central Laboratory For Aquaculture Research In Cooperation With Worldfish Cairo, Egypt, 20-22 Nov.
- Abdelhamid , A.M. (1991). Farm Animals Husbandry. Dar Al-Nasher for Universities (Cairo)/Al-Wafaa Library (Mansourah), Deposit No. 7136/1990, 977-15-0018-x, 429p. (An Arabic textbook).
- Abdelhamid , A.M. (2009). Fundamentals of Fish Production and Culture. The New Universal Office. Alexandria, Deposit No. 24400/2008, 977-438-052-5, 654p. (An Arabic textbook).
- Abdelhamid, A.M. (2019a). The Modern Methods of Aquaculture. Deposition No. 25438/2018, 718p (An Arabic Textbook).
- Abdelhamid, A.M. (2019b). The Blue Revolution. Deposition No. 25443/2018, 743p (An Arabic Textbook).
- Abdelhamid, A.M.; Mehrim, A.I., Abdel Hamid, A.A.M. and Alkatan, M.A.M. (2015). Influence of feeding fish the dried-treated sewage on physiological responses and histological structure of the liver, Asian Journal of Animal and Veterinary Advances, 10 (7): 295-310.
- Abdelhamid, A.M.; Refaey, M.M., Salem, M.F. and Mostafa A. M. El-Kattan, M.A.M. (2019a). Factors affecting fish blood profile: A- Effect of nutritional treatments. Egyptian Journal of Aquatic Biology & Fisheries, 23 (2): 517-526.
- Abdelhamid, A.M.; Refaey, M.M., Salem, M.F. and Mostafa A. M. El-Kattan, M.A.M. (2019b). Factors affecting fish blood profile: B- Effect of environmental and genetic factors. Egyptian Journal of Aquatic Biology & Fisheries, 23 (2): 443-459.
- Abdel-Moneim, A.M.; Amina E Essawy, A.E., Nariman K Badr El-Din, N.K. and Nahed M El-Naggar, N.M. (2013). Biochemical and histopathological changes in liver of the Nile tilapia from Egyptian polluted lakes. Toxicology and Industrial Health, https://doi.org/10.1177/0748233713503374.
- Decie, S. I. V. and Lewis, S. M. (2006). Practical Hematology. 10th Ed., Churchill Livingstone, London. ISBN: 13: 978- 443, PP: 736.
- Dominguez, M.; Takemura, A., Tsuchiya, M. and Nakamura, S. (2004). Impact of different environmental factors on the circulating immunoglobulin levels in the Nile tilapia, Oreochromis niloticus. Aquaculture, 241: 491-500.
- Duncan, D. (1955). Multiple range and multiple F-tests. Biometrics, 11: 1-42.
- Ellis, A. E. (1981). Stress and the modulation of defense mechanisms in fish. In: “Stress and Fish” (A. D. Pickering; Ed.); pp. 147–169. Academic Press; London.
- Hassanen, G.D.I. and Abd Elnabi, H.E. (2017). Physiological evaluation and histological examination of red tilapia fingerlings exposed to different levels of ammonia. 1st Inter. Conf. on "Sustainable Development of Aquaculture" The Central Laboratory For Aquaculture Research In Cooperation With Worldfish Cairo, Egypt, 20-22 Nov.
- Helal, E.G.E.; Abd El-Atti, M. S. and Yasmina, M.Ekraim, Y.M. (2018). Harmful effects of water pollution on some physiological responses of the Nile tilapia (Oreochromis niloticus) in both Qarun and Burullus Lakes. The Journal of Hospital Medicine, 72 (2): 4021-4025.
- Magnadóttir, B. (2006). Innate immunity of fish (overview). Fish & Shellfish Immunology, 20: 137-151.
- McGowan, M.W.; Artiss, J.D., Standbergh, D.R. and Zak, B.A. (1983). Peroxidase-coupled method for colorimetric determination of serum triglycerides. Clin. Chem., 29: 538.
- Natt, M.P. and Herrick, C.A. (1952). A new blood diluent for counting erythrocytes and leucocytes of the chicken. Poultry Science, 31: 735–738.
- Penzlin, H. (1977). Lehrbuch der Tierphysiologie. 2. Auflage, Veb Gustav Fischer Verlag Jena.
- Press, C.McL. and Evensen, O. (1999). The morphology of the immune system in teleost fishes. Fish & Shellfish Immunology, 9: 309-318.
- SAS (2006). SAS statistical guide for personal computer, SAS Institute Inc. Cary, NC.
- Tietz, N.W. (1986). Textbook of clinical chemistry. W.B. Saunders, Philadelphia, 1271.
- Tietz, N.W. (1990). Clinical Guide to Laboratory Tests. 2nd ed. Philadelphia.
- Wotton, I.D. and Freeman, H. (1982). Microanalysis in Medical Biochemistry. Churchill, New York, USA.
Related topics:
Authors:
Mansoura University, Egypt
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.