Aerobic deterioration of silage: causes and controls
Published: August 22, 2007
By: DAVID R. DAVIES, RHUN FYCHAN and RAYMOND JONES (Courtesy of Alltech Inc.)
The practice of making sweet ensilage, the favoured method of the early protagonists, involved allowing the forage in the silo to heat to a temperature above 160 oF (71 oC; Fry, 1885). This practice, which would today be met with horror by the farming community, was promoted well into the 20th century by respected bodies such as the Ministry of Agriculture and Fisheries (Anon., 1926).
Sweet ensilage (with its high level of undesirable volatile fatty acids) is probably also responsible for the fact that aerobic deterioration of silage, as we would recognize it today, was not encountered as a problem until the 1960s or even early 1970s. In support of this view, a review of losses during ensilage by the highly respected researchers McDonald and Whittenbury in 1967 makes no mention of losses during the aerobic feed-out phase.
Twenty-first century silage-making practices have improved greatly since those dark days of the last century and with those improvements the problems have also changed.
Silages are increasingly more nutritious and the fermentation process is more controlled; this in turn has led to aerobic deterioration becoming a major problem, resulting in a post-storage reduction in silage quality due to the growth of yeasts and moulds that can lead to losses of feed dry matter (DM) being as high as 25% in bunker silos (Woolford, 1978; 1990). In the UK alone this spoilage has been estimated to cost the farming industry £110 million per annum (Williams et al., 1995).
The ensilage process
In order to indicate how we have come, in 30 short years or so, from having few or no problems with aerobic deterioration, to a position where it now represents, worldwide, a multi-million dollar cost to the livestock industry, we need to understand a little about the ensilage process.
The ensilage process can be divided into four distinct phases: the initial aerobic phase; the fermentation phase; the stable or storage phase; and a second aerobic phase when the silo is opened. Each phase has consequences for the quality of the product that is fed to livestock. Central to ensilage are the energy fractions; namely the water-soluble carbohydrates (WSC), starch and lactic acid that fuel the microbial processes, which in turn are ultimately responsible for most of the beneficial and detrimental biochemical changes within the silo.
AEROBIC PHASE
A major factor influencing the efficiency with which forages are conserved as silage is the degree of anaerobiosis achieved in the silo (Woolford, 1990). Initially, air trapped in the herbage matrix fuels aerobic processes, including undesirable bacterial and fungal activity. Good ensiling practice, which includes fine chopping and compaction of herbage to exclude air and rapid sealing of the silo, will shorten the initial aerobic phase (McDonald et al., 1991). It will also reduce the chances of large fungal populations developing and compromising the aerobic stability and mycotoxin content of the product.
FERMENTATION PHASE
A number of both facultative and strictly anaerobic microorganisms that enter the silo on the forage may be involved in the fermentation, and thus a diverse range of products can accumulate in silage. During the early stages of fermentation there is competition for substrate (crop WSC) between the facultative and strictly anaerobic microorganisms.
The most important of the bacterial groups are the lactic acid bacteria (LAB) as they ferment WSC to lactic acid, which achieves the primary objective of the fermentation, i.e., a rapid reduction in the pH. The lowering of the pH inhibits the growth of undesirable bacteria such as Enterobacteria and Clostridia, which, if allowed to persist for extended periods of time, ultimately lead to silages with poorer nutritive value (Woolford, 1984; McDonald et al., 1991). The Propionibacteria have occasionally been isolated from silages (deMan, 1957; Woolford, 1975a) but are not normally major participants in the silage fermentation. However, they have the potential to reduce moulds in silages.
Finally there are the fungi, which grow either as single cells, the yeasts, or as multicellular filamentous colonies, the moulds (Woolford, 1984). Until fairly recently there was a general lack of interest in yeasts as they were considered to be a minority group and not major participants in the fermentation. However, under certain conditions very high counts have been observed in, for example, grass silages treated with high levels of formic or sulphuric acid (Henderson et al., 1972; Chamberlain and Quig, 1987) and in silage that has been prepared from wilted forage (Henderson et al., 1972; Jonsson et al., 1990) or where the initial aerobic phase was prolonged (Ruxton and McDonald, 1975). Their activity in silage is not considered to be desirable, as their main fermentation product is ethanol, which contributes little to the preservation process.
However, they can also remain dormant during the storage phase and proliferate greatly during the feed-out phase and, as such, are a main contributor to aerobic deterioration.
The moulds are generally only associated with silage where air has penetrated, such as at the sides and surface of the clamp or bale, and the conditions associated with wellpreserved silage, i.e., low pH and anaerobiosis, are unfavourable for their growth.
THE STABLE OR STORAGE PHASE
In the phase between the end of fermentation and feed-out, providing anaerobic conditions are maintained and a sufficiently low pH within the silage mass has been achieved, few if any changes occur in the silage. However, where air ingress occurs, substantial mould growth is possible with associated mycotoxin production.
THE FEED-OUT PHASE AND AEROBIC DETERIORATION
At feed-out, when air gains access to a hitherto anaerobic environment, well-preserved, high quality silages, with high concentrations of lactic acid and residual WSC or starch, are often prone to spoilage (Honig and Woolford, 1980; Woolford, 1984, 1986; Kennedy, 1990).
There is some evidence to suggest that the susceptibility of silage to aerobic deterioration is higher in inoculant-treated grass (Kennedy 1990; Wyss 1993a) and cereal silages (Honig et al., 1992; Sanderson, 1993; Weinberg et al., 1993; Wyss, 1993b) than in corresponding non-inoculated silages, where hetero-fermentative lactic acid bacteria and other less desirable microorganisms have presumably played a greater role in the fermentation. This higher susceptibility may in part be related to the fact that the production of volatile fatty acids (Woolford, 1975b; 1978) and antimycotic compounds such as ammonia (Woolford, 1984; Mason et al., 1987) and carbon dioxide (Lindgren and Dobrogosz, 1990) is suppressed by directing the fermentation towards lactic acid production using inoculants.
The main microbial groups that are associated with aerobic spoilage include the yeasts (Jonsson and Pahlow, 1984), moulds (Holden, 1987; McDonald et al., 1991), Bacillus species (Woolford, 1978) and acetic acid bacteria (Spoelstra et al., 1988), with pathogenic members of the genus Listeria proliferating at low oxygen concentrations and as the silage pH rises (Fenlon et al., 1989). Populations of these different groups of microorganisms develop successionally and the order can vary depending on the temporal changes in silage composition and environmental conditions.
However, it is generally accepted that while other microorganisms are involved, yeasts play the major role in initiating the aerobic spoilage of silage causing a rise in temperature (Woolford et al., 1982; Jonsson and Pahlow, 1984). Moreover, Woolford (1990) claimed that it is mainly the size and composition of the population of lactate-utilizing yeasts that determine whether a silage will deteriorate upon exposure to air. Yeasts with this characteristic can be undetectable on the crop at harvest but after less than one day of wilting in the field can reach a population size of 104 colony forming units (CFU)/g fresh matter (Jonsson and Pahlow, 1984). Those found in silage are generally divided into two groups according to substrate utilisation and are either saccharolytic or lactate-fermenting (Moon and Ely, 1979; Jonsson and Pahlow, 1984; Middelhoven and Franzen, 1986; McDonald et al., 1991).
On the other hand, despite these distinctions between groups according to classical laboratory tests, it has been suggested that, if examined under conditions similar to those found in silage, all yeasts can use lactate as a carbon and energy source (Middelhoven and Franzen, 1986).
Although there is evidence to suggest that silage inoculants decrease the aerobic stability of silage, aerobic spoilage of good quality silage is a major source of loss to farmers irrespective of whether an additive has been used (Woolford, 1978).
More research is needed on the microbial ecology and population development in aerobically deteriorating silage in order to define clearly the conditions that predispose silages to spoilage of this type. Initially there is a rise in the yeast population followed by an increase in mould numbers during the later stages of deterioration; both population increases are accompanied by a temperature rise, with the second rise also being associated with large increases in pH (Barry et al., 1980; Holden, 1987; Ohyama et al., 1977). The rise in mould growth and concomitant production of mycotoxins has implications for both human and animal health (Oldenburg, 1991).
Thus, it is evident that naturally-occurring silage fermentation is an uncontrolled process.
One of the major causes is variation in numbers and types of lactic acid bacteria and other microorganisms entering the silo (Rooke, 1990; Merry et al., 1993; Davies et al., 1996a). Consequently, a large number of silage additives have been developed to control and improve on the predictability of the fermentation (Anon., 2001). With the gradual increase in the popularity of silage, research has been concentrated on manipulation of the fermentation to improve its quality.
Paradoxically, although this has led to improvements in silage quality, its instability after the silo is opened has become a significant problem. However, air, or more correctly oxygen, is the cause. Woolford (1986) described the presence of air as the ‘Achilles heel’ of the ensilage process with its inextricable link to aerobic deterioration and the losses of silage dry matter and of silage chemical and hygienic quality.
Control of aerobic deterioration
It is therefore clear that aerobic deterioration is of great significance in silage production and a huge problem to the industry. The main protagonists of the problem are the yeasts, with a lesser role for the acetic acid bacteria and moulds. These undesirable microorganisms are present on the crop during growth and harvesting, and poor silage management can lead to a proliferation of these microorganisms in the silo, resulting in a huge population ready to explode upon exposure of the silage face to air.
It is also becoming increasingly evident that one of the consequences of improving silage quality is the negative impact that its enhanced nutritional characteristics may have on aerobic stability. This subject has been reviewed on a number of occasions (Woolford, 1990; McDonald et al., 1991), and it is accepted that well-preserved, high quality silages, particularly those inoculated with homo-fermentative lactic acid bacteria, are more prone to spoilage than untreated silages (Weinberg et al., 1993), due both to the fact that more nutrients are preserved in the silage and there are fewer secondary end products that inhibit the growth of spoilage microorganisms.
Aerobic spoilage of silage can affect both the efficiency of nutrient utilisation (through respiration of energetic fractions) and its hygienic quality (through production of mycotoxins by spoilage microflora).
Thus, we now examine the potential mechanisms available either now or in the future, to control aerobic deterioration and to assess how these may impact silage nutritive value. The methods of control fall into two categories: either through the plant material that is to be ensiled, or through the use of new improved additives that could be based on chemicals, inoculants, or a mixture of both, or novel plant extracts with antimicrobial properties.
CHEMICALS
Organic acids, in particular formic acid, have been widely used as silage additives since the 1950s (Woolford, 1984). Since this time much research has examined the effectiveness of various organic acids on inhibiting aerobic spoilage (Jones et al., 1974; Woolford 1975b; Driehuis et al., 1995).
More recently, Davies et al. (1996b) concluded that a number of lactic acid bacterial inoculants were considerably less effective at reducing aerobic spoilage in maize silage than an additive containing a mix of mainly formic acid and low concentrations of longer chain volatile fatty acids. However, the move towards biological as opposed to chemical silage additives, due to environmental and health and safety concerns of the latter, has resulted in increased interest in developing biological approaches to tackle the problem of aerobic deterioration.
INOCULANTS
To date, most silage inoculants have been developed for their ability to promote a beneficial fermentation that maximizes the nutritive value of the silage for ruminant livestock. For these reasons, they have been based on homo-fermentative lactic acid bacteria that produce lactic acid as the main end product of their fermentation, and, as such, have improved the readily available energy and true protein content of silages (Jones, 1998; Davies et al., 2005).
However, the requirement to reduce aerobic deterioration has a different target because lactic acid is less inhibitory to the yeast population than other fermentation end products and/or by-products. A few microbial approaches have been tried, such as natural end products of microbial fermentation (Grinstead and Barefoot, 1992) or yeast killer toxins (Lowes et al., 2000). However, two more widely utilized approaches have been the use of either propionic acid bacteria, producing predominantly propionic acid as the major end product or hetero-fermentative lactic acid bacteria that produce end products such as acetic acid as well as lactic acid.
PROPIONIC ACID BACTERIA AND THEIR ROLE IN SILAGE
The production of propionic acid during the silage fermentation is unlikely to be detrimental to the fermentation process or to the nutritive value of the silage. In fact, the contrary may be true as there could be a number of additional benefits (Bullerman and Berry, 1966; Langsrud et al., 1978). There are fewer than 20 published reports on propionic acid-producing bacteria and their activities in silage, and Dawson et al. (1993) have previously concluded that the “published research concerning the use of propionic acid-producing bacteria as silage inoculants is lacking and practically devoid.”
This situation has only improved slightly in the last 15 or so years. Publications range from the isolation of propionic acid-producing bacteria from silage (deMan, 1957; Dawson et al., 1992) to their inclusion in silage inoculants (Weinberg et al., 1995a,b). Weinberg et al. (1996) observed that “although the production of propionic acid during the fermentation phase is a sound concept, results in a limited number of controlled experiments have been inconsistent.” Thus, it is fair to conclude, as did Merry and Davies (1999), that current problems in methodology need to be addressed in order to find an ideal organism adapted to the silage ecosystem for use as a silage inoculant.
When seeking a suitable lactic acid bacterial strain for a silage inoculant, Wieringa (1961) isolated 81 strains, but only one had the required characteristics. It is doubtful whether this number of Propionibacteria have ever been isolated from silages and examined for potential as silage inoculants, although recent molecular technological advances may provide a wider range of more appropriate strains (Romanov et al., 2004).
HETERO-FERMENTATIVE LACTIC ACID BACTERIA AND THEIR ROLE IN SILAGE
The shift away from homo-fermentative lactic acid bacteria towards hetero-fermentative lactic acid bacteria began in the late 1990s and the bacterium of choice has been Lactobacillus buchneri (Oude Elferink et al., 1999; Driehuis et al., 2001). This approach has been shown to inhibit the aerobic deterioration of silages through the production of end products other than lactic acid. Products such as acetic acid and 1,2 propanediol are known to be produced (McDonald et al., 1991).
However, whilst this approach can inhibit aerobic deterioration of silage (Driehuis et al., 2001), this effect is not always consistent (Kleinschmit and Kung, 2006). There is considerable debate, within both the research and industry arenas, regarding the validity of using hetero-fermentation due to the compromise made to silage quality and dry matter losses.
It is well documented (Woolford, 1990; McDonald et al., 1991) that the production of acetic acid results in a slower fermentation and thus will probably have a concomitant effect on the protein quality of the silage (Davies et al., 1998; Jones, 1998). In addition to this, acetic acid production in the silo is known to increase dry matter losses and reduce palatability (Woolford, 1990).
In a comparative trial with crimped grain as the material to be ensiled, three different species of hetero-fermentative silage inoculant bacteria were compared with a chemical additive. The chemical treatment had the greatest effect on inhibiting aerobic deterioration whilst in this particular study dry matter losses were lowest in the inoculated treatments (Adesogan et al., 2003); within the trial unfortunately no comparisons were made to the homo-fermentative inoculants.
COMBINATION OF HOMO-FERMENTATIVE LAB INOCULANTS WITH CHEMICAL SALTS
The problems associated with both acid additives and their perceived risks, and the issues of the total effectiveness across the entire ensilage process of the inoculant approaches discussed above, has led to the development of what are frequently termed ‘combination products.’ This approach has combined traditional silage microbiology (homo-fermentative inoculants) with compounds traditionally used in the human food industry (e.g., potassium and sodium salts of sorbate and benzoate) for their antimicrobial activity, particularly against yeasts and moulds (Woolford, 1975c). A few products of this type are now available on the world market.
A number of studies have examined the effect these types of additive have on ensilage (Owen, 2002; Rammer et al., 1999; White et al., 2002). These studies and unpublished research conducted at our Institute have shown improvements in aerobic stability, without the serious negative effects on the silage fermentation associated with some of the other approaches highlighted earlier.
The results of one such trial conducted at IGER are shown in Table 1 and Figure 1. In this study maize (with a composition of 27.4% DM, 9.9% crude protein, 5.9% WSC and 0.7 digestibility) was ensiled either untreated or treated with an inoculant (Sil- All4x4®, Alltech Inc.) or an inoculant plus a stabilizer containing a mixture of sorbate and benzoate salts (Fireguard™, Alltech Inc.) and five replicated samples of each treatment were ensiled in polythene bags in 10 kg bin silos.
After a 100-day ensiling period the silos were opened, silage removed, thoroughly mixed and sub-sampled for chemical analysis by NIRS and aerobic stability measured by monitoring temperature increases in silage in insulated containers maintained at 20 oC ambient temperature. The results indicate that the silage fermentation was similar irrespective of treatment. However the Fireguard™ treatment had significantly improved aerobic stability taking 30 and 25 hrs longer to heat to 2 oC above ambient than the untreated and inoculated silages, respectively.
Table 1. Silage chemical composition and aerobic stability.
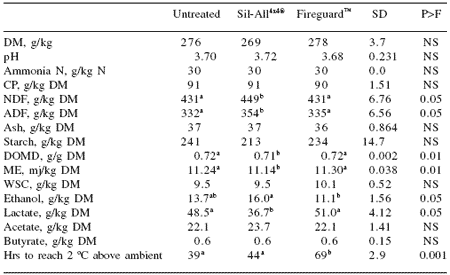
abMeans with different letters within rows differ
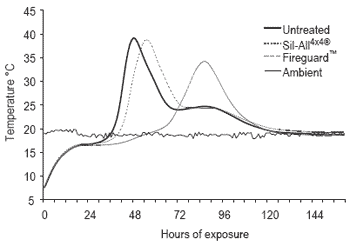
Figure 1. Temperature profiles of silages on exposure to air.
However, the use of these products must come with a note of caution that the chemical inhibitor has activity against all microbial groups including the lactic acid bacteria carrying out the fermentation. Thus all combinations of bacterial strain(s) versus concentration(s) of chemical inhibitor(s) must be tested to ensure that the resultant additive maintains the desired effect of improving both silage fermentation and controlling silage aerobic deterioration.
THE ROLE OF THE PLANT IN INHIBITING AEROBIC DETERIORATION
Despite these combination products appearing to be the solution, there must be continued research to advance our control of the ensilage process further. Certain plant characteristics could make a positive contribution towards limiting aerobic spoilage in the silo.
Lucerne silage has been shown to be more stable than maize silage (Muck and O’Kiely, 1992; O’Kiely and Muck, 1992) and our observations indicate that legume silages (e.g., red clover, lucerne and white clover) are more stable compared with grass silage (R. J. Dewhurst, personal communication), suggesting that legumes may contain natural compounds that inhibit spoilage microorganisms.
However, Muck and O’Kiely (1992) compared the aerobic stability of fresh and ensiled lucerne and concluded that the factor causing stability was produced during ensilage, as the fresh crop was not stable. A greater understanding of the factors involved may present opportunities for breeding or genetic manipulation of these and other forages to enhance aerobic stability.
Another alternative approach could be the stay-green trait. The stay-green trait in grasses is a natural mutation that prevents or delays chlorophyll from progressing through the normal catabolic pathways upon senescence and cell death (Thomas and Smart, 1993) and although not considered in the literature, this behaviour may have application in reducing aerobic spoilage in the silo.
A strong inverse relationship has been observed between different maize varieties that possess increasing resistance to the onset of senescence and accumulate different concentrations of the mycotoxin, zearalenone (Oldenburg, 1999). These observations suggest that the retarded senescence afforded by the stay-green maize cultivars in the sample set provided a less accessible supply of nutrients and consequently supported a smaller epiphytic bacterial and fungal flora. As fungal populations in the silo are the main instigator of aerobic spoilage, reducing the population size of these organisms on the crop is likely to reduce or remove the problem.
Further research is needed to establish a relationship between the stay-green trait in maize and reduced aerobic spoilage in maize silage and whether the relationship is also evident in stay-green grass silages.
Sweet ensilage (with its high level of undesirable volatile fatty acids) is probably also responsible for the fact that aerobic deterioration of silage, as we would recognize it today, was not encountered as a problem until the 1960s or even early 1970s. In support of this view, a review of losses during ensilage by the highly respected researchers McDonald and Whittenbury in 1967 makes no mention of losses during the aerobic feed-out phase.
Twenty-first century silage-making practices have improved greatly since those dark days of the last century and with those improvements the problems have also changed.
Silages are increasingly more nutritious and the fermentation process is more controlled; this in turn has led to aerobic deterioration becoming a major problem, resulting in a post-storage reduction in silage quality due to the growth of yeasts and moulds that can lead to losses of feed dry matter (DM) being as high as 25% in bunker silos (Woolford, 1978; 1990). In the UK alone this spoilage has been estimated to cost the farming industry £110 million per annum (Williams et al., 1995).
The ensilage process
In order to indicate how we have come, in 30 short years or so, from having few or no problems with aerobic deterioration, to a position where it now represents, worldwide, a multi-million dollar cost to the livestock industry, we need to understand a little about the ensilage process.
The ensilage process can be divided into four distinct phases: the initial aerobic phase; the fermentation phase; the stable or storage phase; and a second aerobic phase when the silo is opened. Each phase has consequences for the quality of the product that is fed to livestock. Central to ensilage are the energy fractions; namely the water-soluble carbohydrates (WSC), starch and lactic acid that fuel the microbial processes, which in turn are ultimately responsible for most of the beneficial and detrimental biochemical changes within the silo.
AEROBIC PHASE
A major factor influencing the efficiency with which forages are conserved as silage is the degree of anaerobiosis achieved in the silo (Woolford, 1990). Initially, air trapped in the herbage matrix fuels aerobic processes, including undesirable bacterial and fungal activity. Good ensiling practice, which includes fine chopping and compaction of herbage to exclude air and rapid sealing of the silo, will shorten the initial aerobic phase (McDonald et al., 1991). It will also reduce the chances of large fungal populations developing and compromising the aerobic stability and mycotoxin content of the product.
FERMENTATION PHASE
A number of both facultative and strictly anaerobic microorganisms that enter the silo on the forage may be involved in the fermentation, and thus a diverse range of products can accumulate in silage. During the early stages of fermentation there is competition for substrate (crop WSC) between the facultative and strictly anaerobic microorganisms.
The most important of the bacterial groups are the lactic acid bacteria (LAB) as they ferment WSC to lactic acid, which achieves the primary objective of the fermentation, i.e., a rapid reduction in the pH. The lowering of the pH inhibits the growth of undesirable bacteria such as Enterobacteria and Clostridia, which, if allowed to persist for extended periods of time, ultimately lead to silages with poorer nutritive value (Woolford, 1984; McDonald et al., 1991). The Propionibacteria have occasionally been isolated from silages (deMan, 1957; Woolford, 1975a) but are not normally major participants in the silage fermentation. However, they have the potential to reduce moulds in silages.
Finally there are the fungi, which grow either as single cells, the yeasts, or as multicellular filamentous colonies, the moulds (Woolford, 1984). Until fairly recently there was a general lack of interest in yeasts as they were considered to be a minority group and not major participants in the fermentation. However, under certain conditions very high counts have been observed in, for example, grass silages treated with high levels of formic or sulphuric acid (Henderson et al., 1972; Chamberlain and Quig, 1987) and in silage that has been prepared from wilted forage (Henderson et al., 1972; Jonsson et al., 1990) or where the initial aerobic phase was prolonged (Ruxton and McDonald, 1975). Their activity in silage is not considered to be desirable, as their main fermentation product is ethanol, which contributes little to the preservation process.
However, they can also remain dormant during the storage phase and proliferate greatly during the feed-out phase and, as such, are a main contributor to aerobic deterioration.
The moulds are generally only associated with silage where air has penetrated, such as at the sides and surface of the clamp or bale, and the conditions associated with wellpreserved silage, i.e., low pH and anaerobiosis, are unfavourable for their growth.
THE STABLE OR STORAGE PHASE
In the phase between the end of fermentation and feed-out, providing anaerobic conditions are maintained and a sufficiently low pH within the silage mass has been achieved, few if any changes occur in the silage. However, where air ingress occurs, substantial mould growth is possible with associated mycotoxin production.
THE FEED-OUT PHASE AND AEROBIC DETERIORATION
At feed-out, when air gains access to a hitherto anaerobic environment, well-preserved, high quality silages, with high concentrations of lactic acid and residual WSC or starch, are often prone to spoilage (Honig and Woolford, 1980; Woolford, 1984, 1986; Kennedy, 1990).
There is some evidence to suggest that the susceptibility of silage to aerobic deterioration is higher in inoculant-treated grass (Kennedy 1990; Wyss 1993a) and cereal silages (Honig et al., 1992; Sanderson, 1993; Weinberg et al., 1993; Wyss, 1993b) than in corresponding non-inoculated silages, where hetero-fermentative lactic acid bacteria and other less desirable microorganisms have presumably played a greater role in the fermentation. This higher susceptibility may in part be related to the fact that the production of volatile fatty acids (Woolford, 1975b; 1978) and antimycotic compounds such as ammonia (Woolford, 1984; Mason et al., 1987) and carbon dioxide (Lindgren and Dobrogosz, 1990) is suppressed by directing the fermentation towards lactic acid production using inoculants.
The main microbial groups that are associated with aerobic spoilage include the yeasts (Jonsson and Pahlow, 1984), moulds (Holden, 1987; McDonald et al., 1991), Bacillus species (Woolford, 1978) and acetic acid bacteria (Spoelstra et al., 1988), with pathogenic members of the genus Listeria proliferating at low oxygen concentrations and as the silage pH rises (Fenlon et al., 1989). Populations of these different groups of microorganisms develop successionally and the order can vary depending on the temporal changes in silage composition and environmental conditions.
However, it is generally accepted that while other microorganisms are involved, yeasts play the major role in initiating the aerobic spoilage of silage causing a rise in temperature (Woolford et al., 1982; Jonsson and Pahlow, 1984). Moreover, Woolford (1990) claimed that it is mainly the size and composition of the population of lactate-utilizing yeasts that determine whether a silage will deteriorate upon exposure to air. Yeasts with this characteristic can be undetectable on the crop at harvest but after less than one day of wilting in the field can reach a population size of 104 colony forming units (CFU)/g fresh matter (Jonsson and Pahlow, 1984). Those found in silage are generally divided into two groups according to substrate utilisation and are either saccharolytic or lactate-fermenting (Moon and Ely, 1979; Jonsson and Pahlow, 1984; Middelhoven and Franzen, 1986; McDonald et al., 1991).
On the other hand, despite these distinctions between groups according to classical laboratory tests, it has been suggested that, if examined under conditions similar to those found in silage, all yeasts can use lactate as a carbon and energy source (Middelhoven and Franzen, 1986).
Although there is evidence to suggest that silage inoculants decrease the aerobic stability of silage, aerobic spoilage of good quality silage is a major source of loss to farmers irrespective of whether an additive has been used (Woolford, 1978).
More research is needed on the microbial ecology and population development in aerobically deteriorating silage in order to define clearly the conditions that predispose silages to spoilage of this type. Initially there is a rise in the yeast population followed by an increase in mould numbers during the later stages of deterioration; both population increases are accompanied by a temperature rise, with the second rise also being associated with large increases in pH (Barry et al., 1980; Holden, 1987; Ohyama et al., 1977). The rise in mould growth and concomitant production of mycotoxins has implications for both human and animal health (Oldenburg, 1991).
Thus, it is evident that naturally-occurring silage fermentation is an uncontrolled process.
One of the major causes is variation in numbers and types of lactic acid bacteria and other microorganisms entering the silo (Rooke, 1990; Merry et al., 1993; Davies et al., 1996a). Consequently, a large number of silage additives have been developed to control and improve on the predictability of the fermentation (Anon., 2001). With the gradual increase in the popularity of silage, research has been concentrated on manipulation of the fermentation to improve its quality.
Paradoxically, although this has led to improvements in silage quality, its instability after the silo is opened has become a significant problem. However, air, or more correctly oxygen, is the cause. Woolford (1986) described the presence of air as the ‘Achilles heel’ of the ensilage process with its inextricable link to aerobic deterioration and the losses of silage dry matter and of silage chemical and hygienic quality.
Control of aerobic deterioration
It is therefore clear that aerobic deterioration is of great significance in silage production and a huge problem to the industry. The main protagonists of the problem are the yeasts, with a lesser role for the acetic acid bacteria and moulds. These undesirable microorganisms are present on the crop during growth and harvesting, and poor silage management can lead to a proliferation of these microorganisms in the silo, resulting in a huge population ready to explode upon exposure of the silage face to air.
It is also becoming increasingly evident that one of the consequences of improving silage quality is the negative impact that its enhanced nutritional characteristics may have on aerobic stability. This subject has been reviewed on a number of occasions (Woolford, 1990; McDonald et al., 1991), and it is accepted that well-preserved, high quality silages, particularly those inoculated with homo-fermentative lactic acid bacteria, are more prone to spoilage than untreated silages (Weinberg et al., 1993), due both to the fact that more nutrients are preserved in the silage and there are fewer secondary end products that inhibit the growth of spoilage microorganisms.
Aerobic spoilage of silage can affect both the efficiency of nutrient utilisation (through respiration of energetic fractions) and its hygienic quality (through production of mycotoxins by spoilage microflora).
Thus, we now examine the potential mechanisms available either now or in the future, to control aerobic deterioration and to assess how these may impact silage nutritive value. The methods of control fall into two categories: either through the plant material that is to be ensiled, or through the use of new improved additives that could be based on chemicals, inoculants, or a mixture of both, or novel plant extracts with antimicrobial properties.
CHEMICALS
Organic acids, in particular formic acid, have been widely used as silage additives since the 1950s (Woolford, 1984). Since this time much research has examined the effectiveness of various organic acids on inhibiting aerobic spoilage (Jones et al., 1974; Woolford 1975b; Driehuis et al., 1995).
More recently, Davies et al. (1996b) concluded that a number of lactic acid bacterial inoculants were considerably less effective at reducing aerobic spoilage in maize silage than an additive containing a mix of mainly formic acid and low concentrations of longer chain volatile fatty acids. However, the move towards biological as opposed to chemical silage additives, due to environmental and health and safety concerns of the latter, has resulted in increased interest in developing biological approaches to tackle the problem of aerobic deterioration.
INOCULANTS
To date, most silage inoculants have been developed for their ability to promote a beneficial fermentation that maximizes the nutritive value of the silage for ruminant livestock. For these reasons, they have been based on homo-fermentative lactic acid bacteria that produce lactic acid as the main end product of their fermentation, and, as such, have improved the readily available energy and true protein content of silages (Jones, 1998; Davies et al., 2005).
However, the requirement to reduce aerobic deterioration has a different target because lactic acid is less inhibitory to the yeast population than other fermentation end products and/or by-products. A few microbial approaches have been tried, such as natural end products of microbial fermentation (Grinstead and Barefoot, 1992) or yeast killer toxins (Lowes et al., 2000). However, two more widely utilized approaches have been the use of either propionic acid bacteria, producing predominantly propionic acid as the major end product or hetero-fermentative lactic acid bacteria that produce end products such as acetic acid as well as lactic acid.
PROPIONIC ACID BACTERIA AND THEIR ROLE IN SILAGE
The production of propionic acid during the silage fermentation is unlikely to be detrimental to the fermentation process or to the nutritive value of the silage. In fact, the contrary may be true as there could be a number of additional benefits (Bullerman and Berry, 1966; Langsrud et al., 1978). There are fewer than 20 published reports on propionic acid-producing bacteria and their activities in silage, and Dawson et al. (1993) have previously concluded that the “published research concerning the use of propionic acid-producing bacteria as silage inoculants is lacking and practically devoid.”
This situation has only improved slightly in the last 15 or so years. Publications range from the isolation of propionic acid-producing bacteria from silage (deMan, 1957; Dawson et al., 1992) to their inclusion in silage inoculants (Weinberg et al., 1995a,b). Weinberg et al. (1996) observed that “although the production of propionic acid during the fermentation phase is a sound concept, results in a limited number of controlled experiments have been inconsistent.” Thus, it is fair to conclude, as did Merry and Davies (1999), that current problems in methodology need to be addressed in order to find an ideal organism adapted to the silage ecosystem for use as a silage inoculant.
When seeking a suitable lactic acid bacterial strain for a silage inoculant, Wieringa (1961) isolated 81 strains, but only one had the required characteristics. It is doubtful whether this number of Propionibacteria have ever been isolated from silages and examined for potential as silage inoculants, although recent molecular technological advances may provide a wider range of more appropriate strains (Romanov et al., 2004).
HETERO-FERMENTATIVE LACTIC ACID BACTERIA AND THEIR ROLE IN SILAGE
The shift away from homo-fermentative lactic acid bacteria towards hetero-fermentative lactic acid bacteria began in the late 1990s and the bacterium of choice has been Lactobacillus buchneri (Oude Elferink et al., 1999; Driehuis et al., 2001). This approach has been shown to inhibit the aerobic deterioration of silages through the production of end products other than lactic acid. Products such as acetic acid and 1,2 propanediol are known to be produced (McDonald et al., 1991).
However, whilst this approach can inhibit aerobic deterioration of silage (Driehuis et al., 2001), this effect is not always consistent (Kleinschmit and Kung, 2006). There is considerable debate, within both the research and industry arenas, regarding the validity of using hetero-fermentation due to the compromise made to silage quality and dry matter losses.
It is well documented (Woolford, 1990; McDonald et al., 1991) that the production of acetic acid results in a slower fermentation and thus will probably have a concomitant effect on the protein quality of the silage (Davies et al., 1998; Jones, 1998). In addition to this, acetic acid production in the silo is known to increase dry matter losses and reduce palatability (Woolford, 1990).
In a comparative trial with crimped grain as the material to be ensiled, three different species of hetero-fermentative silage inoculant bacteria were compared with a chemical additive. The chemical treatment had the greatest effect on inhibiting aerobic deterioration whilst in this particular study dry matter losses were lowest in the inoculated treatments (Adesogan et al., 2003); within the trial unfortunately no comparisons were made to the homo-fermentative inoculants.
COMBINATION OF HOMO-FERMENTATIVE LAB INOCULANTS WITH CHEMICAL SALTS
The problems associated with both acid additives and their perceived risks, and the issues of the total effectiveness across the entire ensilage process of the inoculant approaches discussed above, has led to the development of what are frequently termed ‘combination products.’ This approach has combined traditional silage microbiology (homo-fermentative inoculants) with compounds traditionally used in the human food industry (e.g., potassium and sodium salts of sorbate and benzoate) for their antimicrobial activity, particularly against yeasts and moulds (Woolford, 1975c). A few products of this type are now available on the world market.
A number of studies have examined the effect these types of additive have on ensilage (Owen, 2002; Rammer et al., 1999; White et al., 2002). These studies and unpublished research conducted at our Institute have shown improvements in aerobic stability, without the serious negative effects on the silage fermentation associated with some of the other approaches highlighted earlier.
The results of one such trial conducted at IGER are shown in Table 1 and Figure 1. In this study maize (with a composition of 27.4% DM, 9.9% crude protein, 5.9% WSC and 0.7 digestibility) was ensiled either untreated or treated with an inoculant (Sil- All4x4®, Alltech Inc.) or an inoculant plus a stabilizer containing a mixture of sorbate and benzoate salts (Fireguard™, Alltech Inc.) and five replicated samples of each treatment were ensiled in polythene bags in 10 kg bin silos.
After a 100-day ensiling period the silos were opened, silage removed, thoroughly mixed and sub-sampled for chemical analysis by NIRS and aerobic stability measured by monitoring temperature increases in silage in insulated containers maintained at 20 oC ambient temperature. The results indicate that the silage fermentation was similar irrespective of treatment. However the Fireguard™ treatment had significantly improved aerobic stability taking 30 and 25 hrs longer to heat to 2 oC above ambient than the untreated and inoculated silages, respectively.
Table 1. Silage chemical composition and aerobic stability.

abMeans with different letters within rows differ

Figure 1. Temperature profiles of silages on exposure to air.
However, the use of these products must come with a note of caution that the chemical inhibitor has activity against all microbial groups including the lactic acid bacteria carrying out the fermentation. Thus all combinations of bacterial strain(s) versus concentration(s) of chemical inhibitor(s) must be tested to ensure that the resultant additive maintains the desired effect of improving both silage fermentation and controlling silage aerobic deterioration.
THE ROLE OF THE PLANT IN INHIBITING AEROBIC DETERIORATION
Despite these combination products appearing to be the solution, there must be continued research to advance our control of the ensilage process further. Certain plant characteristics could make a positive contribution towards limiting aerobic spoilage in the silo.
Lucerne silage has been shown to be more stable than maize silage (Muck and O’Kiely, 1992; O’Kiely and Muck, 1992) and our observations indicate that legume silages (e.g., red clover, lucerne and white clover) are more stable compared with grass silage (R. J. Dewhurst, personal communication), suggesting that legumes may contain natural compounds that inhibit spoilage microorganisms.
However, Muck and O’Kiely (1992) compared the aerobic stability of fresh and ensiled lucerne and concluded that the factor causing stability was produced during ensilage, as the fresh crop was not stable. A greater understanding of the factors involved may present opportunities for breeding or genetic manipulation of these and other forages to enhance aerobic stability.
Another alternative approach could be the stay-green trait. The stay-green trait in grasses is a natural mutation that prevents or delays chlorophyll from progressing through the normal catabolic pathways upon senescence and cell death (Thomas and Smart, 1993) and although not considered in the literature, this behaviour may have application in reducing aerobic spoilage in the silo.
A strong inverse relationship has been observed between different maize varieties that possess increasing resistance to the onset of senescence and accumulate different concentrations of the mycotoxin, zearalenone (Oldenburg, 1999). These observations suggest that the retarded senescence afforded by the stay-green maize cultivars in the sample set provided a less accessible supply of nutrients and consequently supported a smaller epiphytic bacterial and fungal flora. As fungal populations in the silo are the main instigator of aerobic spoilage, reducing the population size of these organisms on the crop is likely to reduce or remove the problem.
Further research is needed to establish a relationship between the stay-green trait in maize and reduced aerobic spoilage in maize silage and whether the relationship is also evident in stay-green grass silages.
| Conclusions Although there are no substitutes for good clamp management, additives have been used in an attempt to limit aerobic spoilage in the silo. These have had varying degrees of success and whilst the combination products provide a solution to those farmers that are unable to control aerobic deterioration by good silo management alone, there must be continued research effort to develop the next generation of silage additives. Ideally, we need an additive that has absolutely no risks of negative effects on silage quality and, to these ends, continued research needs to be targeted at the development of homofermentative lactic acid bacteria that produce secondary products effective at inhibiting yeasts, moulds and acetic acid bacteria. Investigation of the properties of plants must also continue. As highlighted by Muck and O’Kiely (1992), there is an interaction between the plant and the fermentation to inhibit aerobic deterioration. It has been known for a number of years that allicin, a chemical constituent of members of the onion family, has antimicrobial activity. However, the stability of this activity is questionable particularly under the conditions in the silo. Thus, collaborative research at the Institute of Grassland and Environmental Research jointly with the University of Wales, Aberystwyth is currently screening novel plant products to assess their efficacy for use as silage additives. |
Acknowledgements
We would like to thank colleagues at IGER for their continued support over the years.
References
Adesogan, A.T., M.B. Salawu, A.Vaughan, A.B. Ross, D.R. Davies and A.E. Brooks. 2003. Effect of Lactobacillus buchneri, L. fermentum, Leuconostoc mesenteroides inoculants or a chemical additive on the fermentation, aerobic stability and nutritive value of crimped wheat grains. J. Dairy Sci. 86:1789-1796.
Anon. 1926. Ensilage. Ministry of Agriculture and Fisheries, Misc. Publications No. 53. His Majesty’s Stationary Office, London, UK.
Anon. 2001. Forage Additives Supplement, Farmers Weekly, 21st November 1997.
Barry, T.N., M.E. di Menna, P.R. Webb and J.N. Parle. 1980. Some observations on aerobic deterioration in untreated silages and in silages made with formaldehydecontaining additives. J. Sci. Food. Agric. 31:133-146.
Bullerman L.B. and E.E. Berry. 1966. Use of cheese whey for vitamin B12 production. Appl. Microbiol. 14:353-355.
Chamberlain, D.G. and J. Quig. 1987. The effects of rate of addition of formic acid and sulphuric acid on the ensilage of perennial ryegrass in laboratory silos. J. Sci. Food. Agric. 38:217-228.
Davies, D.R., R.J. Merry and E.L. Bakewell. 1996a. The effect of timing of slurry application on the microflora of grass, and changes occurring during silage fermentation. Grass Forage Sci. 51:42-51.
Davies, D.R., R.J. Merry, R. Jones and R. Fychan. 1996b. Effect of different additives on the aerobic stability of ensiled whole crop maize. In: Proceedings of the 11th International Silage Conference. University of Wales Aberystwyth, UK. pp. 264-265.
Davies, D.R., R.J. Merry, A.P. Williams, E.L. Bakewell, D.K. Leemans and J.K.S. Tweed. 1998. Nutrition, feeding and calves. Proteolysis during ensilage of forages varying in soluble sugar content J. Dairy Sci. 81:444-453.
Davies, D.R., M.K. Theodorou, A.H. Kingston-Smith and R.J. Merry. 2005. Advances in silage quality in the 21st century. In: Silage Production and Utilisation. IGC 2005 Satellite Workshop, Belfast, 3-6 July 2005. (R.S. Park and M.D. Stronge, eds.) Wageningen Academic Publishers, Wageningen, The Netherlands. pp. 121-133.
Dawson T.E., M.T. Yokoyama and S.R. Rust. 1992. Enrichment, isolation, and characterisation of propionic acid producing bacteria from ensiled feedstuffs. J. Anim. Sci. 70:86.
Dawson T.E., S.R. Rust and M.T. Yohoyama. 1993. Manipulation of silage fermentation and aerobic stability by propionic acid producing bacteria. In: Silage Production from Feed to Animal. NRAES-67. Northeast Regional Agricultural Engineering Service, Syracuse, New York State, USA. pp. 96-105.
deMan, J.C. 1957. Some observations on propionic acid fermentation in silage. Antonie Van Leeuwenhook 23:81-86.
Driehuis F., P.G. Van Wikselaar and A.M. Van Vuuren. 1995. Effect of formic, acetic and propionic acid on preservation and aerobic deterioration of grass silage. Ann. Zootech. 44(Suppl.): 95
Driehuis, F., S.J.W.H. Oude Elferink and P.G. Van Wikselarr. 2001. Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homo-fermentative lactic acid bacteria. Grass Forage Sci. 56:330-341.
Fenlon D.R., J. Wilson and J.R. Weddell. 1989. The relationship between spoilage and Listeria monocytogenes contamination in bagged and wrapped big bale silages. Grass Forage Sci. 44:97-100.
Fry, G. 1885. Sweet Ensilage. Durrant, London.
Grinstead, D.A. and S.F. Barefoot. 1992. Jenseniin G, a heat-stable bacteriocin produced by Propionibacterium jensenii P126. Appl. and Env. Micro. 58:215-220.
Henderson, A.R., P. McDonald and M.K. Woolford. 1972. Chemical changes during the ensilage of wilted grass treated with formic acid. J. Sci. Food Agric. 23:1079-1087.
Holden, A.N.G. 1987. Some effects of silage inoculants on aerobic stability. PhD Thesis, University of Newcastle upon Tyne, UK.
Honig, H. and M.K. Woolford. 1980. Changes in silage on exposure to air. In: Forage Conservation in the 80s. Occas. Symp. No. 11 British Grassland Society, Hurley, Maidenhead. (C. Thomas, ed.) British Grassland Society. pp. 77-87.
Honig, H.H., J.G. Schild and R. Daenicke. 1992. Wirkung eines Impfzusatzes in Maissilage-Garverlauf, Verluste und aerobe Stabilitat. VDLUFA Congress, Gottingen, Germany. pp. 399-402.
Jones G.M., D.N. Mowat, J.I. Elliot and E.T. Moran. 1974. Organic acid preservation of high moisture corn and other grains and the nutritional value: a review. Can. J. Anim. Sci. 54:499-517.
Jones, R. 1998. Bridging the protein gap: Potential of forage crops for UK livestock production. In: Biotechnology in the Feed Industry, Proceedings of Alltech’s 14th Annual Symposium (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, UK. pp. 119-133.
Jonsson, A., H. Lindberg, S. Sundas, P. Lingvall and S. Lindgren. 1990. Effect of additives on the quality of big bale silages. Anim. Feed Sci. Tech. 31:139-155.
Jonsson, A. and G. Pahlow. 1984. Systematic classification and biochemical characterisation of yeasts growing in grass silage inoculated with Lactobacillus cultures. Anim. Res. Dev. 20:7-22.
Kennedy, S.J. 1990. Evaluation of three bacterial inoculants and formic acid as additives for first harvest grass. Grass Forage Sci. 45:281-288.
Kleinschmit, D.H. and L. Kung. 2006. The effects of Lactobacillus buchneri 40788 and Pediococcus pentocaseous R1094 on the fermentation of corn silage. J. Dairy Sci. 89:3999-4004.
Langsrud T., G.W. Reinhold and E.G. Hammond. 1978. Free proline production by strains of propionibacteria. J. Dairy. Sci. 61:303-308.
Lindgren, S.E. and W.J. Dobrogosz. 1990. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol. Rev. 87:149-164.
Lowes, K.F., C.A. Shearman, J. Payne, D. Mackenzie, D.B. Archer, R.J. Merry and M.J. Gasson. 2000. Prevention of spoilage in feed and food by the yeast mycocin HMK. Appl. Env. Micro 66:1066-1076.
McDonald P., A.R. Henderson and S.J.E. Heron. 1991. The Biochemistry of Silage. Chalcombe Publications, Marlow, UK.
McDonald, P. and R. Whittenbury. 1967. Losses during ensilage. In: Fodder Conservation. Occas. Symp. No. 3, British Grassland Society, (R.J. Wilkins, ed.) British Grassland Society, Hurley, Maidenhead, Berks, UK. pp 76-85.
Mason V.C., R.J. Merry, G.D. Braithwaite, J.E. Cook, L. Jones and C.J. Hoadley. 1987. Preservation of whole crop wheat using acid and alkaline additives. In: Proc. 8th Silage Conf., Hurley, Maidenhead, Berks. pp. 191-192.
Merry, R.J., R.F. Cussen-Mackenna and R.F. Jones. 1993. Biological silage additives. Cienc. Invest. Agrar. 20:372-401.
Merry, R.J. and D.R. Davies. 1999. Propionibacteria and their role in the biological control of aerobic spoilage in silage. Lait. 79:149-164.
Middelhoven, W.J. and M.M. Franzen. 1986. The yeast flora of ensiled whole-crop maize. J. Sci. Food Agric. 37:855-861.
Moon N.J. and L.O. Ely. 1979. Identification and properties associated with the aerobic deterioration of wheat and alfalfa silages. Mycopathologia 69:153-156.
Muck, R.E. and P.O’Kiely. 1992. Aerobic deterioration of lucerne (Medicago sativa) and Maize (Zea mays) silages - effects of fermentation products. J. Sci. Food Agric. 59:145-149.
Ohyama, Y., S. Hara and S. Masaki. 1977. The use of caproic acid to prevent aerobic deterioration of silages after opening with special reference to the amounts and time of application. J. Sci. Food Agric. 28:369-374.
O’Kiely, P. and R.E. Muck. 1992. Aerobic deterioration of lucerne (Medicago sativa) and maize (Zea mays) silages - effects of yeasts. J. Sci. Food Agric. 59:139-144.
Oldenburg, E. 1991. Mycotoxins in conserved forage. Landbauforschung Volkenrode, Sonderheft. 123:191-205.
Oldenburg, E. 1999. Fungal secondary metabolites in forages: Occurrence, biological effects and prevention. Landbauforschung Volkenrode, Sonderheft. 206:91-109.
Oude Elferink, S.J.W.H., F. Driehuis, J. Krooneman, J.C. Gottschal and S.F. Spoelstra. 1999. Lactobacillus buchneri can improve the aerobic stability of silage via a novel fermentation pathway: the anaerobic degradation of lactic acid to acetic acid and 1,2- propanediol. In: XII Int. Silage Conf., Uppsala, Sweden. (T. Pauly, ed.) pp. 266-267.
Owen, T.R. 2002. The effects of a combination of a silage inoculant and a chemical preservative on the fermentation and aerobic stability of whole crop cereal and maize silage. In: Proc. XIII Int. Silage Conf., 11–13 September 2002, Auchincruive, Scotland. (L.M. Mechie and C. Thomas, eds.) pp. 196-197.
Rammer, C., P. Lingvall and I. Thylin. 1999. Combinations of biological and chemical silage additives. In: XII Int. Silage Conf., Uppsala, Sweden. (T. Pauly, ed.) pp. 327- 328.
Romanov, M.N., R.V. Bato, M.T. Yokoyama and S.R. Rust (2004). PCR detection and 16S rRNA sequence-based phylogeny of a novel Propionibacterium acidipropionici applicable for enhanced fermentation of high moisture corn. J. Appl. Microbiol. 97:38-47.
Rooke J.A. 1990. The numbers of epiphytic bacteria on grass at ensilage on commercial farms. J. Sci. Food Agric. 51:525-533.
Ruxton, I.B. and P. McDonald. 1975. The influence of oxygen on ensilage. J. Sci. Food Agric. 25:107-115.
Sanderson, M.A. 1993. Aerobic stability and in vitro fibre digestibility of microbially inoculated corn and sorghum silages. J. Anim. Sci. 71:505-514.
Spoelstra S.F., M.G. Courtin and J.A.C. van Beers. 1988. Acetic acid bacteria can initiate aerobic deterioration of maize silage. J. Agric. Sci. 111:127-132.
Thomas, H. and C.M. Smart. 1993. Crops that stay green. Ann. Appl. Biol. 123:193- 219.
Weinberg, Z.G., G. Ashbell, Y. Hen and A. Azrieli. 1993. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silage. J. Appl. Bacteriol. 75:512- 518.
Weinberg, Z.G., G. Ashbell, K.K. Bolsen, G. Pahlow, Y. Hen and Azrieli A. 1995a. The effect of a propionic acid bacterial inoculant applied at ensiling, with or without lactic acid bacteria, on the aerobic stability of pearl-millet and maize silages. J. Appl. Bacteriol. 78:430-436.
Weinberg, Z.G., G. Ashbell, Y. Hen and A. Azrieli. 1995b. The effect of a propionic acid bacterial inoculant applied at ensiling on the aerobic stability of wheat and sorghum silages. J. Ind. Microbiol. 15:493-497.
Weinberg, Z.G. and R.E. Muck. 1996. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 19:53-68.
White, J.S., C.J. Lin, M.K. Woolford and K.K. Bolsen. 2002. A new solution to the old problem of cereal and maize silage stability In: Proc. XIII Silage Conf., Auchincruive, Scotland. pp. 78-79.
Wieringa, G.W. 1961. The influence of green forages on fermentation. Futterkonservierung. 7:27-35.
Williams, A.G., D.L. Critten and A.M. Reynolds. 1995. A mathematical model of the aerobic deterioration of silage. Grass Forage Sci. 50:132-146.
Woolford, M.K. 1975a. The significance of Propionibacterium spp. and Micrococcus lactylyticus to the ensiling process. J. Appl. Bacteriol. 39:301-306.
Woolford, M.K. 1975b. Microbiological screening of the straight chain fatty acids (C1- C12) as potential silage additives. J. Sci. Food Agric. 26:219-228.
Woolford, M.K. 1975c. Microbiological screening of food preservatives, cold sterilants and specific antimicrobial agents as potential silage additives. J. Sci. Food Agric. 26:229-237.
Woolford, M.K. 1978. Aerobic deterioration of silage. ARC Rev. 4:8-12.
Woolford, M.K., K.K. Bolsen and L.S. Peart. 1982. Studies on the aerobic deterioration of whole-crop cereal silages. J. Agric. Sci. (Camb.) 98:529-535.
Woolford, M.K. 1984. The Silage Fermentation. Marcel Dekker, New York, USA.
Woolford, M.K. 1986. Aerobic deterioration of silage. In: Developments in Silage. (B.A. Stark and J.M. Wilkinson, eds.) Chalcombe Publications, Marlow, UK.
Woolford, M.K. 1990. A review: the detrimental effects of air on silage. J. Appl. Bacteriol. 68:101-116.
Wyss, U. 1993a. Einsatz eines milchsaurebakterie-impfzusatzes bei grasssilage aus der sicht der konsevierung. Lanwirtsch.Schweiz 6:202-207.
Wyss, U. 1993b. Einsatz eines milchsaurebakterie-impfzusatzes bei maissilage aus der sicht der konsevierung. Lanwirtsch. Schweiz 6:501-505.
Authors: DAVID R. DAVIES, RHUN FYCHAN and RAYMOND JONES
Institute of Grassland and Environmental Research, Plas Gogerddan, Aberystwyth, Ceredigion, UK
Institute of Grassland and Environmental Research, Plas Gogerddan, Aberystwyth, Ceredigion, UK
Related topics
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post




.jpg&w=3840&q=75)