Introduction
Coccidiosis caused by the apicomplexan protozoan of genus Eimeria (E) is a common and costly diseases associated with poultry production across the world.1,2 There are several species of chicken coccidia, i.e., E. maxima, E. mitis, E. necatrix, E. praecox E. tenella, E. acervulina, E. hagani, E. mivati and E. brunetti. Among the listed species, E. maxima, E. tenella, and E. acervulina are the most common species.3-5 The spread of the disease is through fecal‐oral route, and signs of Eimeria infection include edema of submucosa and hemorrhagic enteritis which is associated with thickening of the intestinal wall and mucin production. Damage caused by Eimeria to the gut epithelial cells results in reduced nutrient absorption, decreased performance, morbidity and mortality.6-9 Use of anticoccidial drugs to control coccidiosis is a common practice in conventional broiler production, but their use has been associated with the development of drug resistance coccidia.10-12 There is also increased pressure for the poultry industry to move away from the use of anticoccidial drugs due to legislative regulation and consumer demands for antibiotic free poultry products.13-15
Use of live vaccines have proven to be important for control of coccidiosis; however, vaccines from different companies have been shown to vary in terms of type of Eimeria species, total number of oocysts and attenuated status of the organism.16,17 Application of Eimeria vaccines use spray cabinets and gels in most hatcheries in the United States. The birds become immune with fecal oral reingestion over time of vaccine derived parasites. A challenge associated with vaccines is that not all antigens provide enough immunity against heterologous species or different strains of the same species.18-20
The definition of a “good vaccine” may differ between stakeholders, and multiple parameters including mortality rate, body weight gain, feed conversion ratio, intestinal gross lesion and microscopic lesion scoring have been used to measure the efficacy of Eimeria vaccines. However, body weight gain is of primary significance in broiler production and microscopic lesion scoring have been shown to detect significant damage caused by varying oocyst dosages.21,22 Variability in results from vaccines challenge studies makes it difficult to compare in part due to differences in experimental design and parameters evaluated. Therefore in this study, efficacy of commonly used live coccidia vaccines (LCV) against field isolates from commercially raised broiler chickens was evaluated.
Materials and methods
Husbandry
A 39 day trial was conducted at the University of Maryland Eastern Shore’s (UMES) poultry research facility under the approval and guidelines of the University’s Animal Care and Use Committee. Two thousand four hundred male broiler chicks and 2,400 female broiler chicks were obtained from a commercial hatchery on hatch day. Two hundred male chicks and two hundred female chicks were placed in one of 12 rooms in the UMES Environmental House so that each room were equally mixed with males and females. Each room contained used pine shaving bedding (approximately 7.62 cm depth) that was a minimum of six flocks old. Chicks were vaccinated for Newcastle Disease and Infectious Bronchitis in the hatchery (no field vaccination), and each room housed 400 birds (1 bird/0.305m2). Each room is 6.096 x 6.096 m and is equipped with commercial pan feeders, nipple drinkers, and thermostat controlled forced air heaters. A standard commercial non-medicated diet was provided. All feed and water were offered ad libitum.
All rooms have independent temperature and ventilation control. The ventilation was controlled by a ten-minute timer in each room. The minimum ventilation followed commercial recommendations21 and was identical for all rooms. As minimum ventilation requirements increased with age, the time set on the timer fans in each room were set identical. Each room was also equipped with a backup thermostat fan which was set to run four degrees above the target temperature. In addition, a standard industry light program was followed.
Immunization
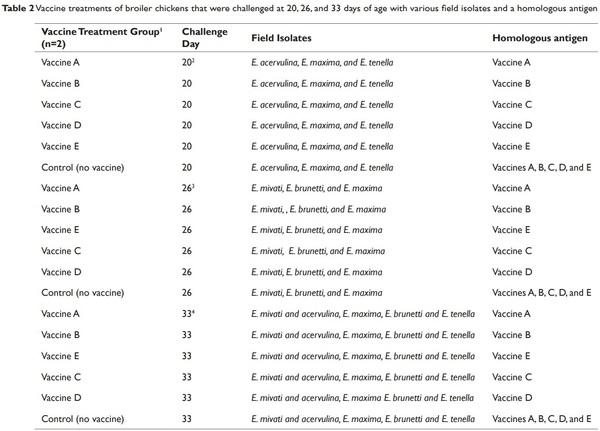
The following LCV experimental treatments (Table 2) were evaluated in this study: Vaccine A (contains live oocysts of E. acervulina, E. maxima, E maxima MFP, E. mivati, and E. tenella), Vaccine B (contains live oocysts of E. acervulina, E. maxima and E. tenella), Vaccine C (contains attenuated species of E. maxima, E. acervulina, and E. tenella), Vaccine D (contains E. acervulina, E. brunetti, E. maxima, E. necatrix and E. tenella) and Vaccine E (contains live oocysts of E. acervulina, E. tenella and E. maxima). The Eimeria species profile of each LCV are declared from each LCV manufacturer.
The six vaccine experimental treatments (Non-vaccinated control, Vaccine A, Vaccine B, Vaccine C, Vaccine D, and Vaccine E) were randomly assigned to rooms so that each treatment was replicated once within each block. Vaccination was performed via coarse spray with a spray cabinet following manufacturer’s recommendations. Each LCV experimental treatment was replicated two times with 400 chicks/replicate. Each room was blocked based on room location and considered an experimental unit. Birds were allowed to stay in the rooms assigned throughout the immunization phase.
Control chickens were provided with an anticoccidial drug (amprolium at 0.012% dosage level via water) to minimize cross contamination. The drug was removed approximately 48 hours prior to each challenge days. Fresh fecal samples were collected on 7, 10, 14, 17, 21, 24 and 27 d of age to determine oocysts output and the point of cessation of oocysts shedding.
Challenge
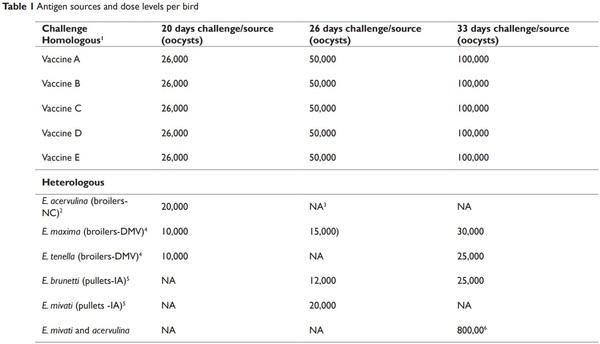
Birds were challenged on 20, 26, and 33 d of age with 3-5 field isolates and a homologous antigen. Four to five birds were challenged for each field isolate and homologous antigen. The field isolates were from coccidia samples from clinical cases from pullets or broiler chickens. The descriptions of the field isolates and homologous antigens challenged for each LCV experimental treatment are provided in Tables 1 and 2.
1 Vaccine A contains live oocysts of E. acervulina, E. maxima, E maxima MFP, E. mivati, and E. tenella, (Merck Animal Health), Vaccine B contains live oocysts of E. acervulina, E. maxima and E. tenella, (Huvepharma, Inc.), Vaccine C contains attenuated species of E. maxima, E. acervulina, and E. tenella, (Boehringer Ingelheim Animal Health), Vaccine D contains oocysts of E. acervulina, E. brunetti, E. maxima, E. necatrix and E. tenella, (Ceva Animal Health, LLC) and Vaccine E contains live oocysts of E. acervulina, E. tenella and E. maxima, (Huvepharma, Inc).
2 Strains obtain from commercial broiler farms in North Carolina.
3 Not applicable, birds were not challenged with this heterologous strain on the designated challenge day.
4 Strains obtain from commercial broiler farms in Delmarva region.
5 Strains obtain from commercial pullet farms in Iowa.
6 40,000 oocysts of E. mivati and 40,000 oocysts of E. acervulina.
1 Two replicates (400 birds/replicate)/ LCV experimental treatment.
2 Five birds were challenged for each field isolate and each homologous antigen (20 birds/LCV replicate and 40 birds/control replicate)
3 Four birds were challenged for each field isolate and each homologous antigen (16 birds/LCV replicate and 32 birds/control replicate)
4 Four birds were challenged for each field isolate and each homologous antigen (20 birds/LCV replicate and 36 birds/control replicate)
On the day of challenge each bird was given 1 ml of one of the field (heterologous strains) or homologous antigen challenge strains via gavage. Each bird was given approximately 10,000-100,000 sporulated oocysts. The dose level was determined by the species of coccidia (Table 1). The challenged birds were housed separately from the other immunized birds.
Evaluation
The efficacy of the vaccines used in this study were evaluated based on microscopic scoring and body weight gain of the challenged birds.
1. Coccidia Microscopic Parasitic Scoring (MS): Between 132 to 144 hr post-challenge, wet mount smears were prepared for all intestines from all challenged birds from each room to determine the level of parasitism using a 0 to 4 system.24 The lesion scorer was blinded to the LCV treatment and challenge antigen group being scored.
2. Bird weights: Birds were group weighed (4-5 birds/Eimeria challenge) on each challenge day and 132-144 hr post challenge to determine average bird gain during the challenge period. Five birds/ Eimeria challenge were weighed on d 20 and d 26. Four birds/Eimeria challenge were weighed on d 26 and d 32 and d 33 and 39.
Statistical analysis
A Randomized Complete Block (RCB) design was used with six LCV experimental treatments and two replicates/treatment. Block was considered a random factor, and vaccine and challenge treatments were fixed effects. Significant differences among treatments means were determined using Tukey’s-HSD test with a 5% level of probability. The dependent variables that were measured were body weight gain and MS. All data were subjected to analysis of variance (ANOVA) as a RCB, using the general linear model procedure of STATISTIX® 10 software (Analytical Software, Tallahassee, FL, USA).
Results
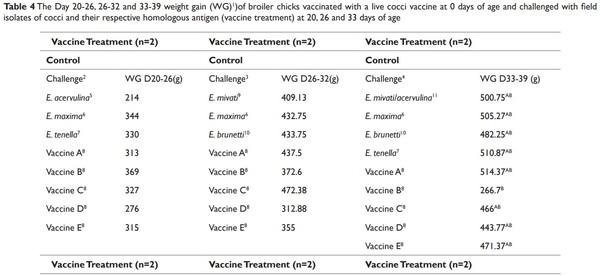
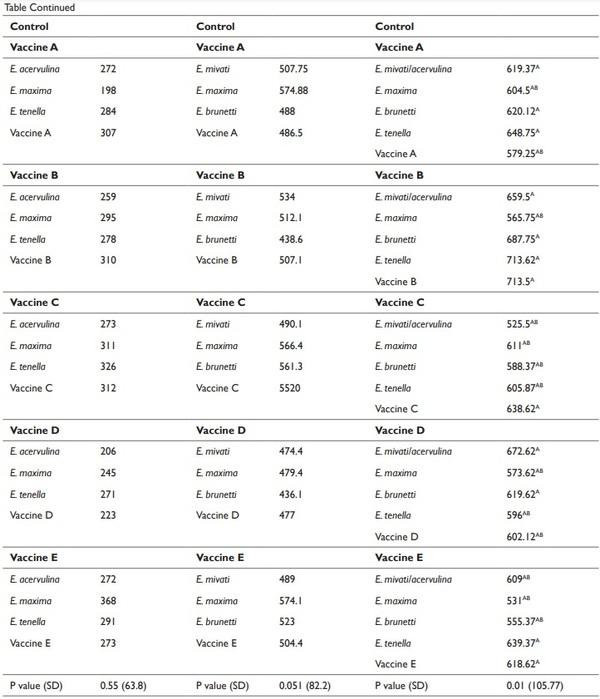
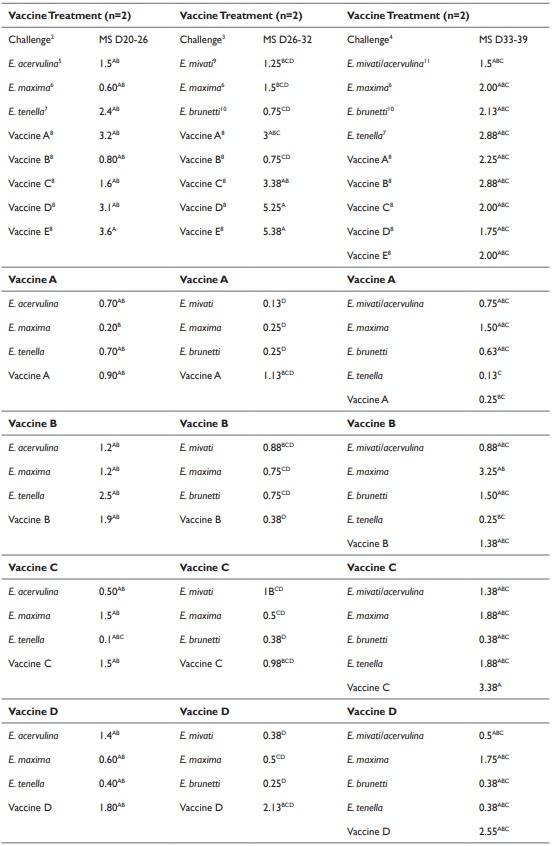
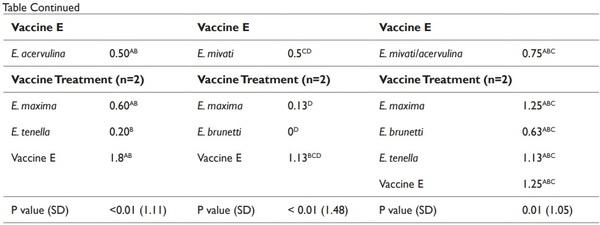
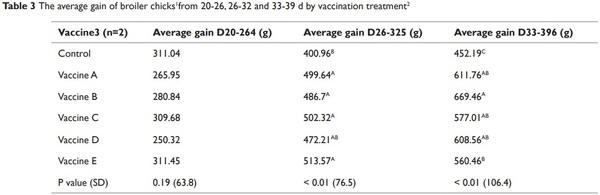
The effect of LCV treatments on the average gain of birds are provided in Table 3. The weight gains of all of the challenged birds/ LCV treatments were averaged to determine the effect of vaccination on bird gain. No differences (P > 0.05) were detected in the d 20 to 26 average gain of broilers between the treatments (Table 3). However differences were detected in the d 26-32 and d 33-39 average gain of broilers (Table 3). The average gain of the non-vaccinated broilers from d 26-32 and d 33-39 was lower (P ≤ 0.05) compared to the gain of broilers from the vaccinated treatments. No differences were detected in the d 26-32 weight gain of birds between the vaccinated treatments. The d 33-39 weight gains of birds vaccinated with vaccine treatments A, B, C, and D were not significantly different from each other (Table 3). The effect of the LCV treatments that were challenged with Eimeria species are presented in Tables 4 and 5. No significant differences in the d 20-26 and 26-32 weight gain were detected between any of the treatments (Table 4). However, the d 33-39 weight gain of birds that were immunized with vaccine B and challenged with the homologous antigen was higher (P ≤ 0.05) compared to the d 33-39 weight gain of the control birds that were challenged with the homologous antigen (713.5 g and 266.7 g, respectively). No significant reductions in the MS of immunized experimental birds challenged on d 20 with E. acervulina, E. maxima, E. tenella, and the homologous antigens were detected when compared to the MS of the control birds challenged on d 20 with E. acervulina, E. maxima, E. tenella and their respective homologous antigens. The MS of the birds from the control vaccine treatment that were challenged with E. tenella at 20 d of age was 2.4. The MS of the birds that were immunized with vaccines C, D, and E and challenged with E. tenella were reduced numerically by 96, 83, and 92%, respectively, however this reduction was not significant (Table 5).
1 Birds were group weighed (4 or 5 birds) prior to each challenge (d 20, 26 and 33). Six d post-challenge, birds were group weighed to determine weight gain during the challenge period. The weight gains of all the challenged birds/LCV treatments were averaged to determine the effect of vaccination on bird gain.
2 Vaccination was performed via coarse spray with a spray cabinet following manufacturer’s recommendations.
3 Manufactured recommended dose was administered for each vaccine
4 Five birds were challenged for each field isolate and each homologous antigen (20 birds/LCV replicate and 40 birds/control replicate)
5 Four birds were challenged for each field isolate and each homologous antigen (16 birds/LCV replicate and 32 birds/control replicate)
6 Four birds were challenged for each field isolate and each homologous antigen (20 birds/LCV replicate and 36 birds/control replicate)
A-CMeans within the same column with no common superscript differ significantly (P ≤ 0.05) according to Tukey’s HSD all pairwise comparison test.
1 Birds were group weighed (4 or 5 birds) prior to each challenge (d 20, 26 and 33). Six d post-challenge, birds were group weighed to determine weight gain during the challenge period.
2 Five birds were challenged for each field isolate and each homologous antigen (20 birds/replicate for each vaccine treatment and 40 birds/replicate for control treatment)
3 Four birds were challenged for each field isolate and each homologous antigen (16 birds/replicate for each vaccinated treatment and 32 birds/replicate for control treatment)
4 Four birds were challenged for each field isolate and each homologous antigen (20 birds/replicate for each vaccinated treatment and 36 birds/replicate for control treatment)
5 Each bird was challenged with 20,000 oocysts.
6 Each bird was challenged with 10,000, 15,000 and 30,000 oocysts on d 20, 26 and 33, respectively.
7 Each bird was challenged with 26,000 and 25,000 oocysts on d 20 and 33, respectively.
8 Each bird was challenged with 26,000, 50,000 and 100,000 oocysts on d 20, 26 and 33, respectively.
9 Each bird was challenged with 20,000 oocysts on d 26.
10 Each bird was challenged with 12,000 and 25,000 oocysts on d 26 and 33, respectively
11Each bird was challenged with 40,000 oocysts of E. mivati and 40,000 oocysts of E. acervulina on d 33.
A-BMeans within the same column with no common superscript differ significantly (P ≤ 0.05) according to Tukey’s HSD all pairwise comparison test.
Table 5 The Day 20-26, 26-32 and 33-39 intestinal microscopic parasitic score (MS1 ) of broiler chicks vaccinated with a live cocci vaccine at 0 days of age and challenged with field isolates of cocci and their respective homologous antigen (vaccine treatment) at 20, 26 and 33 days of age
1 MS were determine six d post-challenge.
2 Five birds were challenged for each field isolate and each homologous antigen (20 birds/replicate for each vaccine treatment and 40 birds/replicate for control treatment)
3 Four birds were challenged for each field isolate and each homologous antigen (16 birds/replicate for each vaccinated treatment and 32 birds/replicate for control treatment)
4 Four birds were challenged for each field isolate and each homologous antigen (20 birds/replicate for each vaccinated treatment and 36 birds/replicate for control treatment)
5 Each bird was challenged with 20,000 oocysts
6 Each bird was challenged with 10,000, 15,000 and 30,000 oocysts on d 20, 26 and 33, respectively.
7 Each bird was challenged with 26,000 and 25,000 oocysts on d 20 and 33, respectively.
8 Each bird was challenged with 26,000, 50,000 and 100,000 oocysts on d 20, 26 and 33, respectively.
9 Each bird was challenged with 20,000 oocysts on d 26.
10 Each bird was challenged with 12,000 and 25,000 oocysts on d 26 and 33, respectively
11Each bird was challenged with 40,000 oocysts of E. mivati and 40,000 oocysts of E. acervulina on d 33.
A-DMeans within the same column with no common superscript differ significantly (P ≤ 0.05) according to Tukey’s all pairwise comparison test.
Similar results of MS were detected from the d 26 and 33 challenges. Although not significant, an 83% and 91% reduction of the MS of birds challenged on d 26 with E. maxima and immunized with vaccines A and E, respectively compared to the MS of control treatment birds challenged with E. maxima. Immunizations with vaccines A, B and D tended also to reduce the MS of d 33 E. tenella challenged birds with compared to the MS of the control birds challenged with E. tenella.
Discussion
Coccocidial vaccines are considered viable means to control the disease.25 However, according to Blake et al.,26 results of the vaccination can be influenced by variation in pathogenicity and immunogenicity of Eimeria species. Use of strain or isolate from the vaccine as a challenge has been suggested as a means to ensure antigenic and species homogeneity. In addition, use of antigenic distinct strains can be used to assess cross protection.27 In this study, the MS of the birds that were immunized with vaccines C, D, and E and challenged with E. tenlla were 96, 83, and 92% numerically lower. Additionally, vaccines A and E numerically reduced (83% and 91%, respectively) the MS of birds challenged on d 26 with E. maxima compared to the MS of the control treatment birds challenged with E. maxima. Immunizations with vaccines A, B and D also tended to reduce the MS of d 33 E. tenella challenged birds with compared to the MS of the control birds challenged with E. tenella. The data suggests that vaccinated birds may have developed protection against secondary infection of coccidiosis based on reduction of MS when compared to the control (Table 5). However, the level of cross protection against various Eimeria species was highly variable. In contrast, Nollet et al.28 reported no difference in the overall MS of chicks that were immunized on d 0 for coccidiosis and challenged at 15 d of age with Eimeria species compared to the MS of chicks that were not immunized and challenged on d 15.
There are multiple parameters used to measure pathogenicity of Eimeria species infections in broiler chickens. Some of these include weight gains, intestinal lesion scores and changes in serum alkaline phosphatase activity.29 Over the years, gross lesion score (GLS) describe by Johnson and Reid,30 have been used to assess effectiveness of Eimeria treatment including vaccines, however this method has been reported to have a wide range of pathologic variation21 and chickens inoculated with same dose of oocysts have been reported to have a large variation in GLS.29 In contrast MS is reported to be a more objective method to detect mucosal damage caused by varying oocyst dosage.22 Idris et al.,22 suggests that MS can detect the presence of coccidiosis infection in broilers that may be overlooked by using GLS alone. The authors reported that E. acervulina infections produce more obvious GLS. However, E. maxima is a more difficult species to perform GL scoring because the level of infections does not always correlate with the severity of the gross lesion scores.30 While presence and or absence of lesions can be used evaluate the efficacy of the vaccines, lesion scores should be correlated with other criteria i.e. performance (weight gain and feed efficiency).31
Coccidian challenge has been reported to have a significant impact on intestinal integrity of broiler chickens which in turn affect nutrient digestibility and absorption. Day 0 vaccinated chicks and challenged at 26 and 33 d of age had higher weight gains compared to the nonvaccinated challenged birds. Crouch et al.,32 reported similar results with vaccinated birds. It was reported, chicks that were vaccinated via spray cabinet at 1 d of age and challenged at 28 d of age with Eimeria species had higher weight gains compared to the weight gains of nonvaccinated challenged birds.32 The vaccinated administered chicks saw no reduction in weight gain due to the coccidiosis challenge. Similar results were reported when birds were inoculated with E. tenella and challenged at 25 d of age with the homologous oocysts,33 moreover a challenge with Eimeria maxima was shown to result in reduced villi height in the jejunum and reduced expression of nutrient transporters located in the brush border of the intestinal epithelial cells.34 Hence higher weight gain in our experiment for the vaccinated birds during challenge can be explained in part due to secondary immunity provided by vaccines during secondary exposure to Eimeria species.35
Conclusion
Immunizing birds using LCV at d 0 resulted in improved weight gain of birds challenged with Eimeria species at 26 and 33 d of age. Although immunizing birds with LCVs did not significantly reduce the MS of challenged birds compared to the controls, the MS tended to be reduced numerically.
This article was originally published in Journal of Microbiology & Experimentation 2021;9(6):186‒194. DOI: 10.15406/jmen.2021.09.00342. This is an Open Access article distributed under the terms of the Creative Commons Attribution License.