I. Introduction
Plant-derived isoquinoline alkaloids (IQ) have the potential to support gut integrity and growth performance under conditions of enhanced stress such as heat stress. The previous study revealed that the BW increased in the IQ group, and the level of FITC-d, to evaluate the gut barrier function, reduced in the IQ supplement group (Kitasato et al., 2021). However, a comprehensive study on the effect of IQ on growth performance and gut integrity under heat stress during the summer season in a tropical climate has not been reported so far. The objective of the present work was to study the impact of feeding IQ on growth performance, inflammatory status, and gut integrity under heat stress conditions in broilers reared during the summer season in a tropical climate.
II. Materials and methods
Ethics approval: MHESI 68014/1571. 720 day-old male Ross 308 broiler chicks were randomly distributed into three treatments: 1) negative control (fed on a basal diet), 2) IQ 60 (supplemented with 60 mg/kg feed, supplemented as Sangrovit® Extra (Phytobiotics Futterzusatzstoffe GmbH, Germany)), 3) IQ 100 (supplemented with 100 mg/kg feed). Each treatment was divided into eight replicates with 30 chickens per replicate. Birds were housed in an open production house in the Southern area of Thailand (April-May). House temperature ranged from 33.0-35.0oC and relative humidity RH ranged from 70-75%. Each replicate was assigned to a wire-floored cage (2.5 x 2.5 m) and equipped with a self-feeder and waterer. All birds were provided ad libitum access to feed and water. Nutrient contents in diets matched the requirements of broilers reared in tropical climates. Birds were fed mash diets throughout the duration of the trial (42 days), split up into a starter (day 1 – 21), grower (day 21 -35), and finisher period (day 35 – 42). Live body weight (BW), body weight gain (BWG), feed intake (FI), feed conversion ratio (FCR), and survival rate were recorded.
On day 35, one chicken was selected from each replicate to determine gut integrity, stress response and inflammatory status. FITC-d was used as a marker to assess gut integrity followed by Vicuña et al. (2015). Real-time quantitative PCR was performed for intestinal tight junction (TJ) proteins, junctional adhesion molecule 2 (JAM-2), Occludin 1, Zonula occludens-1 (ZO-1), Mucin 2 (MUC-2), and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Serum cortisol and serotonin levels were determined by HPLC method, according to Theapparat et al. (2017) and Chen et al. (2015). In addition, cytokine gene expression in the ileum was analyzed for IFN-γ, IL-4, TNFα, iNOS, NF-κB, according to Livak and Schmittgen (2001).
III. Results and discussion
a) Performance parameters
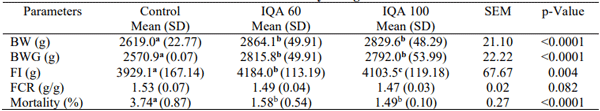
The average FI, BW, BWG, FCR, and mortality of broilers fed IQ are presented in Table 1 for the overall trial period. BW, BWG, FI, and survival rate were statistically significant different for broilers fed diets supplemented with IQ at 60 and 100 mg/kg feed compared with the control treatment (P < 0.05). A trend for an improved FCR was observed for birds fed IQ (P < 0.1). The current results are in accordance with Kikusato et al. (2021). The authors fed IQ to birds, induced artificial heat stress, and observed a positive impact of IQ on performance parameters.
Table 1 - Effect of isoquinoline alkaloids (IQ) on growth performance parameters of broiler chickens from 1 to 42 days of age.
b) Serum fluorescein isothiocyanate-dextran (FITC-d), corticosterone, and serotonin levels
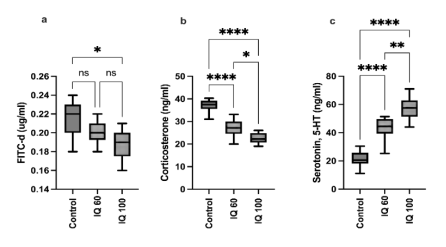
Supplementation of 100 ppm IQ significantly decreased (P < 0.05) FITC-d levels in the blood (Figure 1a), pointing out a positive impact on gut integrity. IQ supplementation tended to influence corticosterone levels (Figure 1b), implying a lower stress response in IQ-fed birds. Glucocorticoid secretion is well known to be controlled by the hypothalamic-pituitary-adrenal axis (HPA), and cytokines are known to stimulate the hypothalamic-pituitary-adrenal axis function (Herman et al., 2016). The activation of the HPA axis leads to increased levels of corticosterone, which may result in feed intake depression, and consequently weight loss in poultry (Johnson, 2002). In accordance, serotonin-5HT levels were significantly increased in the IQ supplemented groups when compared to the control (Figure 1c).
Figure 1 - a) Fluorescein isothiocyanate-dextran (FITC-d), b) Corticosterone, and c) Serotonin levels in serum of heat-stressed broiler chickens at 35 days of age from three treatments; basal diet (control), isoquinoline alkaloids at 60 mg/kg (IQ 60), or 100 mg/kg diet (IQ 100). Different superscripts indicate significant differences; * (P < 0.05), ** (P < 0.01), ****(P < 0.0001).
c) The gene expression of intestinal inflammation and tight junction proteins
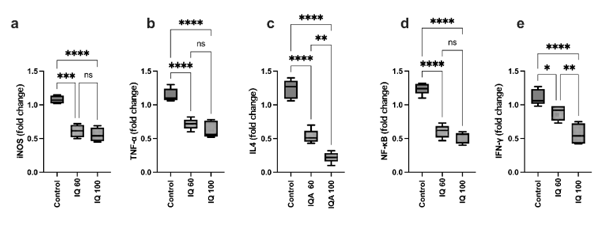
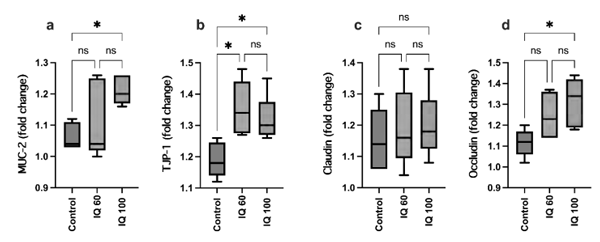
The supplementation of IQ at 60 and 100 mg/kg feed significantly decreased (P < 0.05) the expression of iNOS, TNF-α, IL-4, IFN-γ, and NF-κB, in the ileum of heat-stressed broiler chickens compared to the control (P < 0.0001). While for the expression of IL-4 and IFN-γ, a dose-dependent effect of IQ-supplementation could be observed, this effect could not be observed for iNOS, TNFα, and NF-κB (Figure 2). Chaturvedi et al. (1997) and Soler et al. (2016) reported that IQs have an impact on NF-κB, IL-1β, or TNF-α levels. In addition, IQ decreased the expression of iNOS (Khadem et al., 2014; Kisusato et al., 2020). Birds fed 100 mg IQ/kg feed showed a significantly increased level of TJP-1, MUC-2, and Occludin in the jejunum compared to control birds, while the lower inclusion rate of 60 mg IQ/kg feed only had a significant impact on TJP-1 (Figure 3).
Figure 2 - The gene expression of iNOS, TNF-α, IL-4, IFN-γ, NF-κB, in the ileum of broilers under heat-stress conditions at 35 days of age; basal diet (control), isoquinoline alkaloids at 60 mg/kg (IQ 60), or 100 mg/kg diet (IQ 100). Different superscripts indicate significant differences; * (P < 0.05), ** (P < 0.01), ***(P < 0.001), ****(P < 0.0001).
Figure 3 - The gene expression of MUC-2, TJP-1, Claudin, Occludin, in the jejunum of broiler reared under heat stress conditions at 35 days of age; basal diet (control), isoquinoline alkaloids at 60 mg/kg (IQ 60) or 100 mg/kg diet (IQ 100). Different superscripts indicate significant differences; * (P < 0.05).
IV. Conclusions
In conclusion, the current study showed that broilers reared under heat-stress conditions in a tropical climate are subjected to inflammation which in turn has a negative impact on gut integrity and therefore performance. Isoquinoline alkaloids offer support to birds under heat-stress conditions by alleviating the negative consequences of heat-stress, therefore contributing to an economical and sustainable broiler production.
Presented at the 33th Annual Australian Poultry Science Symposium 2022. For information on the next edition, click here.