Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease
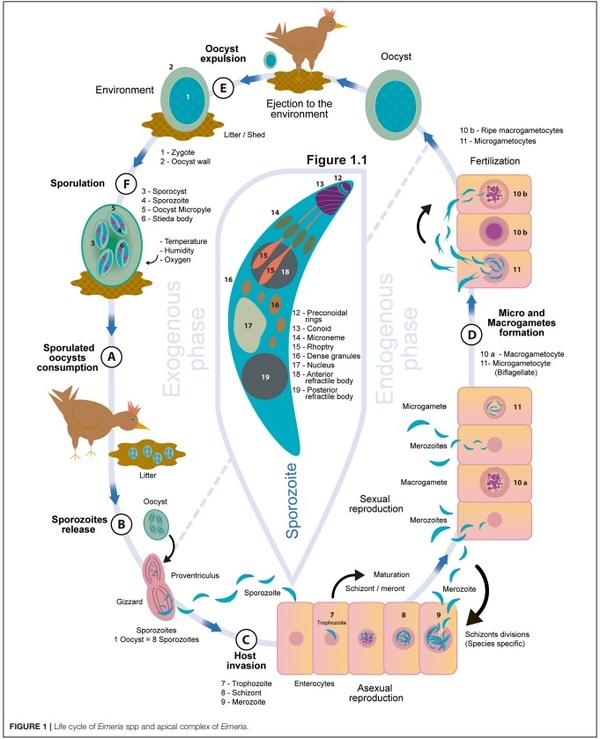
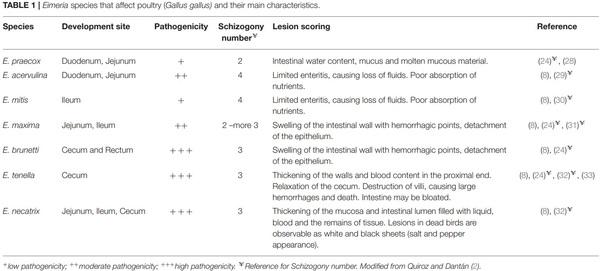
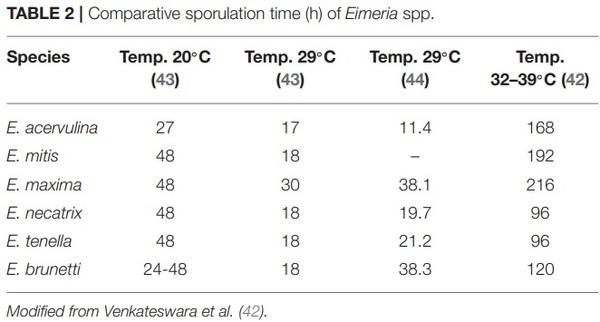
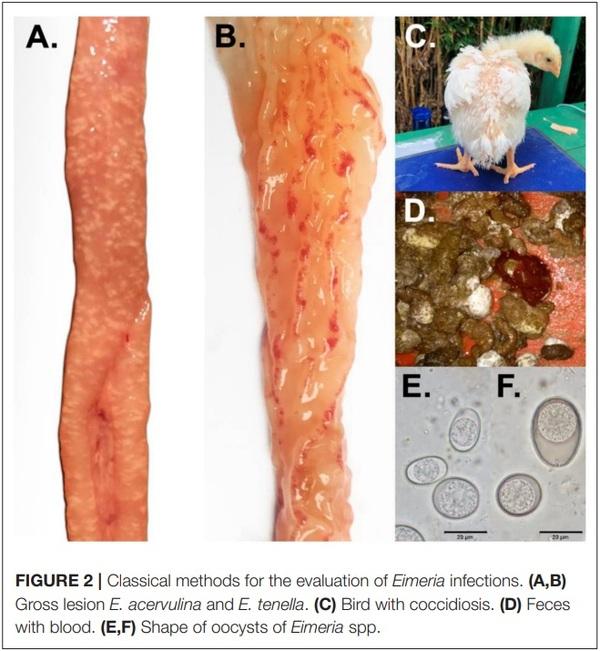
1. Bogosavljevic-Boskovic S, Mitrovic S, Djokovic R, Doskovic V, Djermanovic
V. Chemical composition of chicken meat produced in extensive indoor and free range rearing systems. Afr J Biotechnol. (2010) 9:9069–75. doi: 10.5897/AJB10.1084
2. Quiroz-Castañeda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. BioMed Res Int. (2015) 2015:430610. doi: 10.1155/2015/430610
3. USDA. Livestock and Poultry: World Markets and Trade. United States
Department of Agriculture Foreign Agricultural Service January 10, 2020. (2020). Available at: https://apps.fas.usda.gov/psdonline/circulars/livestock_ poultry.pdf. (accessed: March 10, 2021).
4. O’neill BC, Dalton M, Fuchs R, Jiang L, Pachauri S, Zigova K. Global demographic trends and future carbon emissions. Proc. Natl. Acad. Sci. (2010) 107:17521–26. doi: 10.1073/pnas.1004581107
5. Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, et al. Food security: the challenge of feeding 9 billion people. Science. (2010)
327:812–18. doi: 10.1126/science.1185383
6. Cervantes HM, McDougald L.R, Jenkins MC. “Coccidiosis,” In: Diseases of
Poultry, Volume II. Fourteenth Edition. Editor-in-chief David E. Swayne:
John Wiley & Sons, Inc. (2020). p. 1193–217.
7. Vrba V, Blake DP, Poplstein M. Quantitative real-time PCR assays for detection and quantification of all seven Eimeria species that infect the chicken. Vet Parasitol. (2010) 174:183–90. doi: 10.1016/j.vetpar.2010.09.006
8. Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci J. (2014) 93:501–11. doi: 10.3382/ps.2013-03634
9. Shivaramaiah C, Barta JR, Hernandez-Velasco X, Téllez G, Hargis BM.
Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet Med Res Rep. (2014) 5:23–34. doi: 10.2147/VMRR.S57839
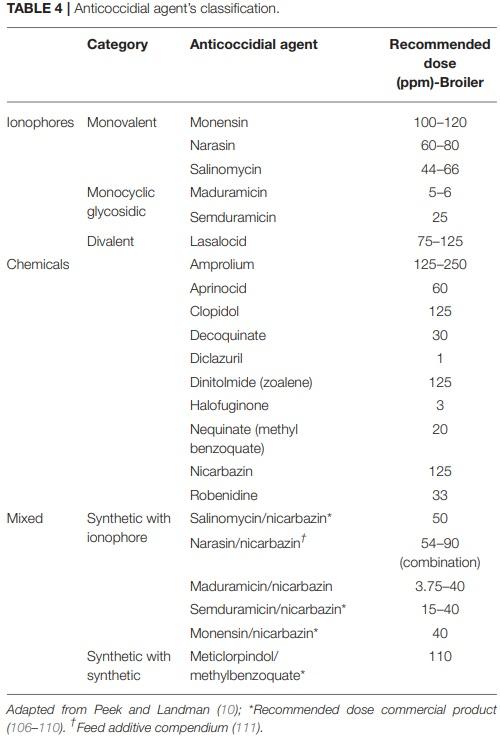
10. Peek HW, Landman WJM. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q. (2011) 31:143–61. doi: 10.1080/01652176.2011.605247
11. Abbas RZ, Iqbal Z, Blake D, Khan MN, Saleemi MK. Anticoccidial drug resistance in fowl coccidia: the state of play revisited. World Poult Sci J. (2011)
67:337–50. doi: 10.1017/S004393391100033X
12. Chapman HD, Jeffers TK. Restoration of sensitivity to salinomycin in
Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poult Sci J. (2015)
94:943–46. doi: 10.3382/ps/pev077
13. Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol. (1999)
29: 1209–29. doi: 10.1016/S0020-7519(99)00086-7
14. Müller J, Hemphill A. In vitro culture systems for the study of apicomplexan parasites in farm animals. Int J Parasitol. (2013) 43:115– 24. doi: 10.1016/j.ijpara.2012.08.004
15. Suarez CE, Bishop RP, Alzan HF, Poole WA. Cooke BM. Advances in the application of genetic manipulation methods to apicomplexan parasites. Int
J Parasitol. (2017) 47:701–10. doi: 10.1016/j.ijpara.2017.08.002
16. Levine ND. Protozoan Parasites of Domestic Animals and Man. Minneapolis:
Burgess Publishing Company. (1961).
17. Williams RB. Review Article: anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. (2002) 31:317–53. doi: 10.1080/03079450220148988
18. Haug A, Gjevre AG, Skjerve E, Kaldhusdal M, A. survey of the economic impact of subclinical Eimeria infections in broiler chickens in Norway. Avian Pathol. (2008) 37:333–41. doi: 10.1080/030794508020
50705
19. Khazandi M. Title The Eimeria-Host Cell Interaction in Broiler Chickens.
Roseworthy, SA: University of Adelaide. (2006).
20. Nabian S, Arabkhazaeli F, Seifouri P, Farahani A. Morphometric analysis of the intestine in experimental coccidiosis in broilers treated with anticoccidial drugs. Iran. J. Parasitol. (2018) 13:493–99.
21. Madlala T, Okpeku M, Adeleke MA. Understanding the interactions between
Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review. Parasite. (2021) 28:1–10. doi: 10.1051/parasite/2021047
22. Bowman D. Georgis’Parasitology for Veterinarians. St Louis, MO:
Elsevier. (2014).
23. Chapman HD. Studies on the excystation of different species of eimeria in vitro. Zeitschrift Parasitenkd Parasitol Res. (1978) 56:115–21. doi: 10.1007/BF00930742
24. Kheysin YM. Life Cycles of Coccidia of Domestic Animals. New York, NY:
Elsevier. (1972).
25. Rose ME, Lawn AM, Millard BJ. The effect of immunity on the early events in the life-cycle of Eimeria tenella in the caecal mucosa of the chicken. Parasitology. (1984) 88:199–210. doi: 10.1017/S0031182000054470
26. Trout JM, Lillehoj HS. Eimeria acervulina infection: evidence for the involvement of CD8+ T lymphocytes in sporozoite transport and host protection. Poult Sci. (1995) 74:1117–25. doi: 10.3382/ps.0741117
27. Tierney J, Mulcahy G. Comparative development of Eimeria tenella (Apicomplexa) in host cells in vitro. Parasitol Res. (2003) 90:301–4. doi: 10.1007/s00436-003-0846-1
28. Long PL, Reid WM. Guide for the diagnosis of coccidiosis in chickens. The
University of Georgia College of agricultura experiment station research report. (1982), 404.
29. Vetterling JM, Doran DJ. Schizogony and gametogony in the life cycle of the poultry coccidium, Eimeria acervulina Tyzzer, 1929. J Parasitol. (1966)
12:1150–57. doi: 10.2307/3276360
30. Novilla MN, Jeffers TK, Griffing WJ, White SL. A redescription of the life cycle of Eimeria mitis Tyzzer, 1929. J Protozool. (1987) 34: 87–92. doi: 10.1111/j.1550-7408.1987.tb03139.x
31. Dubey JP, Jenkins MC. Re-evaluation of the life cycle of Eimeria maxima Tyzzer, 1929 in chickens (Gallus domesticus). Parasitology. (2018) 145:1051–58. doi: 10.1017/S00311820170
02153
32. McDonald V, Rose ME. Eimeria tenella and E. necatrix: a third generation of schizogony is an obligatory part of the developmental cycle. J Parasitol. (1987) 17:617–22. doi: 10.2307/3282145
33. Duffy CF, Mathis GF, Power RF. Effects of NatustatTM supplementation on performance, feed efficiency and intestinal lesion scores in broiler chickens challenged with Eimeria acervulina, Eimeria maxima and
Eimeria tenella. Vet Parasitol. (2005) 130:185–90. doi: 10.1016/j.vetpar.2005.
03.041
34. Ahmad TA, El-Sayed BA, El-Sayed LH. Development of immunization trials against Eimeria spp. Trials Vaccinol. (2016) 5:38–47. doi: 10.1016/j.trivac.2016.02.001
35. Walker RA, Ferguson DJ, Miller CM, Smith NC. Sex and
Eimeria: a molecular perspective. Parasitology. (2013) 140:1701–17. doi: 10.1017/S0031182013000838
36. Tewari AK, Maharana BR. Control of poultry coccidiosis: changing trends. J
Parasit Dis. (2011) 35:10–7. doi: 10.1007/s12639-011-0034-7
37. Lee DL, Millard BJ. The structure and development of the macrogamete and oocyst of Eimeria acervulina. Parasitology. (1971) 62:31–34. doi: 10.1017/S0031182000071262
38. Scholtyseck E, Mehlhorn H, Hammond DM. Electron microscope studies of microgametogenesis in coccidia and related groups. Z Parasitenkd. (1972)
38:95–131. doi: 10.1007/BF00329023
39. You MJ. The comparative analysis of 618 infection pattern and oocyst output in Eimeria tenella, E. maxima and E acervulina in young broiler chicken Vet
World. (2014) 7:542–7. doi: 10.14202/vetworld.2014.542-547
40. Waldenstedt L, Elwinger K, Lunden A, Thebo P, Uggla A. Sporulation of
Eimeria maxima oocysts in litter with different moisture contents. Poult Sci. (2001) 80:1412–15. doi: 10.1093/ps/80.10.1412
41. Awais MM, Akhtar M, Iqbal Z, Muhammad F, Anwar MI. Seasonal prevalence of coccidiosis in industrial broiler chickens in Faisalabad,
Punjab, Pakistan. Trop Anim Health Pro. (2011) 44:323–28. doi: 10.1007/s11250-011-0024-x
42. Venkateswara RP, Raman M, Gomathinayagam S. Sporulation dynamics of poultry Eimeria oocysts in Chennai. J Parasit Dis. (2015) 39:689–92. doi: 10.1007/s12639-013-0403-5
43. Edgar SA. Sporulation of oocysts at specific temperatures and notes on the prepatent period of several species of avian coccidia. J Parasitol. (1955)
41:214–16. doi: 10.2307/3273795
44. Norton CC, Chard MJ. The oocyst sporulation time of Eimeria species from the fowl. Parasitol. (1983) 86:193–8. doi: 10.1017/S0031182000050368
45. Reid WM, Long PL. A diagnostic chart for nine species of fowl coccidian.
Georgia Agric. exp. Stn. Tech. Bull. Editor N. B. Bowen, Athen: College of
Agriculture, University of Georgia (1979). p 5–24.
46. Arabkhazaeli F, Nabian S, Modirsaneii M, Mansoori B, Rahbari S.
Biopathologic characterization of three mixed poultry Eimeria spp. isolates.
I Iran J Parasitol. (2011) 6:23.
47. Györke A, Pop L, Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. (2013) 20:50. doi: 10.1051/parasite/2013052
48. Kant V, Singh P, Verma PK, Bais I, Parmar MS, Gopal A. et al.
Anticoccidial drugs used in the poultry: an overview. Sci Int. (2013) 1:261–65. doi: 10.17311/sciintl.2013.261.265
49. Conway DP, McKenzie ME. Poultry Coccidiosis: Diagnostic and Testing
Procedures. London: John Wiley & Sons. (2007).
50. Morris GM, Woods WG, Richards DG, Gasser RB. Investigating a persistent coccidiosis problem on a commercial broiler–breeder farm utilising
PCR-coupled capillary electrophoresis. Parasitol Res. (2007) 101:583–89. doi: 10.1007/s00436-007-0516-9
51. Cantacessi C, Riddell S, Morris GM, Doran T, Woods WG. Otranto,
D, Gasser RB. Genetic characterization of three unique operational taxonomic units of Eimeria from chickens in Australia based on nuclear spacer ribosomal DNA. Vet Parasitol. (2008) 152:226–34. doi: 10.1016/j.vetpar.2007.12.028
52. Blake DP, Vrba V, Xia D, Jatau ID, Spiro S, Nolan MJ, Tomley FM, et al.
Genetic and biological characterisation of three cryptic Eimeria operational taxonomic units that infect chickens (Gallus gallus domesticus). Int J
Parasitol. (2021) 51:621–34. doi: 10.1016/j.ijpara.2020.12.004
53. Joyner LP, Long PL. The specific characters of the Eimeria, with special reference to the coccidia of the fowl. Avian Pathol. (1974) 3:145–57. doi: 10.1080/03079457409353827
54. Williams RB. Effects of different infection rates on the oocyst production of Eimeria acervulina or Eimeria tenella in the chicken. Parasitology. (1973)
67:279–88. doi: 10.1017/S0031182000046515
55. Williams RB. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J
Parasitol. (2001) 31: 1056–69. doi: 10.1016/S0020-7519(01)00235-1
56. Jenkins MC. Dubey, JP, Miska K, Fetterer R. Differences in fecundity of
Eimeria maxima strains exhibiting different levels of pathogenicity in its avian host. Vet Parasitol. (2017) 236:1–6. doi: 10.1016/j.vetpar.2017.01.009
57. Lillehoj HS, Lillehoj EP. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis. (2000) 44:408–25. doi: 10.2307/1592556
58. Lillehoj HS. Influence of inoculation dose, inoculation schedule, chicken age, and host genetics on disease susceptibility and development of resistance to
Eimeria tenella infection. Avian Dis. (1988) 32:437–44. doi: 10.2307/1590909
59. Hadas G, Mebrhatu G. Abebe T. Prevalence of poultry coccidiosis in
Gondar town, North West Ethiopia Am Eurasian. J Agric Environ Sci. (2014) 9:129–35. doi: 10.5829/idosi.aejsr.2014.9.5.86147
60. Wondimu A, Mesfin E, Bayu Y. Prevalence of poultry coccidiosis and associated risk factors in intensive farming system of Gondar Town,
Ethiopia. Vet Med Int. (2019) 2019:1–6. doi: 10.1155/2019/5748690
61. Greenacre CB, Morishita TY. Backyard Poultry Medicine and Surgery: A
Guide for Veterinary Practitioners. Hoboken, NJ : John Wiley & Sons. (2021).
62. Peek HW. Resistance to Anticoccidial Drugs: Alternative Strategies to Control
Coccidiosis in Broilers, Doctoral dissertation, Utrecht University. (2010).
63. Ontario Ministry of Agriculture. Managing Coccidiosis in My Poultry Flock.
Availale online at: https://atrium.lib.uoguelph.ca/xmlui/bitstream/handle/
10214/11932/ManagingCoccidiosisInMyPoultryFlock.pdf?sequence=3. (accessed July 2021).
64. Carvalho FS, Wenceslau AA, Teixeira M, Carneiro JAM, Melo ADB.
Albuquerque GR. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet Parasitol. (2011) 176:95–100. doi: 10.1016/j.vetpar.2010.11.015
65. Barrios MA, Da Costa M, Kimminau E, Fuller L, Clark S, Pesti G, et al.
Relationship between broiler body weights, Eimeria maxima gross lesion scores, and microscores in three anticoccidial sensitivity tests. Avian Dis. (2017) 61:237–41. doi: 10.1637/11518-102116-Reg.1
66. Hauck R, Carriosa M, McCrea BA, Dormitorio T, Macklin KS. Evaluation of next-generation amplicon sequencing to identify Eimeria spp. of chickens.
Avian Dis. (2019) 63:577–83. doi: 10.1637/aviandiseases-D-19-00104
67. Hinsu AT, Thakkar JR, Koringa PG, Vrba V, Jakhesara SJ, Psifidi A, et al.
Illumina next generation sequencing for the analysis of Eimeria populations in commercial broilers and indigenous chickens. Front Vet Sci. (2018) 5:176. doi: 10.3389/fvets.2018.00176
68. Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. (2000) 24:303–24. doi: 10.1016/S0145-305X(99)00080-4
69. Ali H, Naqvi F, Tariq N. Prevalence of coccidiosis and its association with risk factors in poultry of Quetta, Pakistan. Asian J Appl Sci. (2014) 2:4.
70. Adams C, Vahl HA. Veldman A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: development of an experimental infection model. Br J Nutr. (1996) 75:867–73. doi: 10.1079/BJN199
60192
71. Assis RCL, Luns FD, Beletti ME, Assis RL, Nasser NM, Faria ESM, et al. Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeria acervulina. Vet Parasitol. (2010) 168:185–9. doi: 10.1016/j.vetpar.2009.11.017
72. Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie
RI, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol
Immunopathol. (2008) 122:104–15. doi: 10.1016/j.vetimm.2007.10.014
73. Al-Quraishy S, Qasem MA, Al-Shaebi EM, Murshed M, Mares MM, Dkhil
MA. Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella. J King Saud Univ Sci. (2020) 32:2207-11. doi: 10.1016/j.jksus.2020.02.034
74. Pearson JP, Brownlee IA. Structure and function of mucosal surfaces. Coloniz
Mucos Surf. (2005) 13:3–16. doi: 10.1371/journal.pone.0030287
75. Montagné L, Piel C. Lallès JP. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr Rev. (2004) 62:105–14. doi: 10.1111/j.1753-4887.2004.tb00031.x
76. Sharma R, Schumacher U. Morphometric analysis of intestinal mucins under different dietary conditions and gut flora in rats. Digest Dis Sci. (1995)
40:2532–39. doi: 10.1007/BF02220438
77. Meslin JC, Fontaine N. Andrieux C. Variation of mucin distribution in the rat intestine, caecum and colon: effect of the bacterial flora. Comp Biochem Physiol Mol Integr Physiol. (1999) 123:235–39. doi: 10.1016/S1095-6433(99)00056-2
78. Al-Sheikhly F, Al-Saieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. (1980) 24:324–33. doi: 10.2307/1589700
79. Adhikari P, Kiess A, Adhikari R, Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poultry Res. (2020)
29:515–34. doi: 10.1016/j.japr.2019.11.005
80. Ruff MD. Important parasites in poultry production systems. Vet
Parasitol. (1999) 84:337–347. doi: 10.1016/S0304-4017(99)00076-X
81. Khater HF, Ziam H, Abbas A, Abbas RZ, Raza MA, Hussain K, et al. Avian coccidiosis: recent advances in alternative control strategies and vaccine development. Agrobiol Rec. (2020) 1:11–25. doi: 10.47278/journal.abr/2020.004
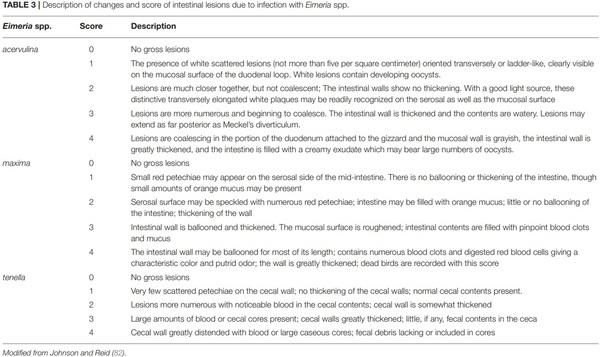
82. Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. (1970)
28:30–6. doi: 10.1016/0014-4894(70)90063-9
83. Price KR. Use of live vaccines for coccidiosis control in replacement layer pullets. J Appl Poultry Res. (2012) 21:679–92. doi: 10.3382/japr.2011-00486
84. Raman M, Banu SS, Gomathinayagam S, Raj GD. Lesion scoring technique for assessing the virulence and pathogenicity of Indian field isolates of avian
Eimeria species. Vet Arh. (2011) 81:259–71.
85. Hodgson JN. Coccidiosis: Oocyst counting technique for coccidiostat evaluation. Exp Parasitol. (1970) 28:99–102. doi: 10.1016/0014-4894(70)90073-1
86. Bortoluzzi C, Paras KL, Applegate TJ, Verocai GG. Comparison between
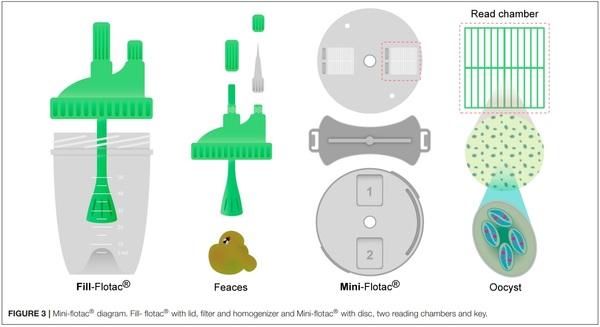
Mcmaster and mini-FLOTAC methods for the enumeration of Eimeria maxima oocysts in poultry excreta. Vet Parasitol. (2018) 254:21–5. doi: 10.1016/j.vetpar.2018.02.039
87. Cringoli G, Maurelli MP, Levecke B, Bosco A, Vercruysse J, Utzinger J, et al. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat Protoc. (2017) 12:1723. doi: 10.1038/nprot.2017.067
88. Silva LMR, Vila-Viçosa MJM, Maurelli MP, Morgoglione ME, Cortes HCE,
Cringoli G, et al. Mini-FLOTAC for the diagnosis of Eimeria infection in goats: an alternative to McMaster. Small Ruminant Res. (2013) 114:280–83. doi: 10.1016/j.smallrumres.2013.06.017
89. Noel ML, Scare JA, Bellaw JL, Nielsen MK. Accuracy and precision of miniFLOTAC and McMaster techniques for determining equine strongyle egg counts. J Equine Vet Sci. (2017) 48:182–87. doi: 10.1016/j.jevs.2016.09.006
90. Da¸s G, Klauser S, Stehr M, Tuchscherer A. Metges CC. Accuracy and precision of McMaster and Mini-FLOTAC egg counting techniques using egg-spiked faeces of chickens and two different flotation fluids. Vet Parasitol. (2020) 283:109158. doi: 10.1016/j.vetpar.2020.109158
91. You MJ. Detection of four important Eimeria species by multiplex PCR in a single assay. Parasitol Int. (2014) 63:527–32. doi: 10.1016/j.parint.2014.01.006
92. Hamidinejat H, Shapouri MS, Mayahi M, Borujeni, MP. Characterization of Eimeria species in commercial broilers by PCR based on ITS1 regions of rDNA. Iran J Parasitol. (2010) 5:48–54.
93. Kumar S, Garg R, Moftah A, Clark EL, Macdonald SE, Chaudhry AS, et al.
An optimised protocol for molecular identification of Eimeria from chickens.
Vet Parasitol. (2014) 199:24–31. doi: 10.1016/j.vetpar.2013.09.026
94. Tang X, Huang G, Liu X, El-Ashram S, Tao G, Lu C, et al. An optimized DNA extraction method for molecular identification of coccidian species. Parasitol
Res. (2018) 117:655–64. doi: 10.1007/s00436-017-5683-8
95. Shirley MW. Bumstead N. Intra-specific variation within Eimeria tenella detected by the random amplification of polymorphic DNA. Parasitol Res. (1994) 80:346–51. doi: 10.1007/BF02351878
96. Fernandez S, Katsuyama AM, Kashiwabara AY, Madeira AMB, Durham
AM, Gruber A. Characterization of SCAR markers of Eimeria spp. of domestic fowl and construction of a public relational database (The Eimeria SCARdb). FEMS Microbiol Lett. (2004) 238:183–88. doi: 10.1016/j.femsle.2004.07.034
97. Shu-San L, Lik-Sin L, Efendi NA, Blake DP, Kawazu SI, Wan KL. Comparison of molecular methods for the detection of Eimeria in domestic chickens in Malaysia. Sains Malays. (2019) 48:1425–32. doi: 10.17576/jsm-2019-4
807-11
98. Barkway CP, Pocock RL, Vrba V, Blake DP. Loop-mediated isothermal amplification (LAMP) assays for the species-specific detection of Eimeria that infect chickens. BMC Vet Res. (2011) 7:1–8. doi: 10.1186/1746-6148-7-67
99. Moraes JC, França M, Sartor AA, Bellato V, de Moura AB, Magalhães MDLB, et al. Prevalence of Eimeria spp. in broilers by multiplex PCR in the southern region of Brazil on two hundred and fifty farms. Avian Dis. (2015) 59:277–81. doi: 10.1637/10989-112014-Reg
100. Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Kumar
S, et al. Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int J Parasitol. (2016) 46:537–44. doi: 10.1016/j.ijpara.2016.05.006
101. Chapman HD, Jeffers TK, Williams RB. Forty years of monesin for the control of coccidiosis in poultry. Poult Sci J. (2010) 89:1788–801. doi: 10.3382/ps.2010-00931
102. Chapman HD, A. landmark contribution to poultry science-Prophylactic control of coccidiosis in poultry. Poult Sci. (2009) 88:813–15. doi: 10.3382/ps.2008-00316
103. Poultry Health Today. Nearly 60% of US broilers now raised without antibiotics, but that number may have peaked. (2020). Available online at: https://poultryhealthtoday.com/nearly-60-of-usbbroilers-now-raisedwithout-antibiotics-but-that-number-may-have-peaked/?utm_source=
Poultry$+$Health$+$Today$+$Newsletter&utm_campaign=daab24c737-
AAAP_antimicrobial_stewardship_PHT_1_8_2018_COPY_0&utm_ medium=email&utm_term=0_5ac605299a-daab24c737-315439401. (accessed Sept 2021).
104. Chapman HD, Jeffers TK. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int J Parasitol
Drugs Drug Resist. (2014) 4:214–17. doi: 10.1016/j.ijpddr.2014.10.002
105. Witcombe DM, Smith NC, Hemphill A. Strategies for anticoccidial prophylaxis. Parasitology. (2014) 141:1379–89. doi: 10.1017/S0031182014000195
106. Ilender. Salincarb
R . (2021). Available online at: https://www.ilendercorp. com/productos/#/salinocarb/197/112/1/15. (accessed October 22, 2021).
107. Zoetis. Gromax
R (2013). Available online at: https://www.zoetisus. com/_locale-assets/poultry/poultry-literature-library/apac-en/gromax_ productprofile_zp130181-a_apac-en_zoetis.pdf. (accessed October 22,
2021).
108. Phibro- animal health corporation. Aviax
R Plus (2018). Available online at: https://phibrosaludanimal.com/news/descarga-aviax-plus/folletos/FOLLAviax-Plus-brochure-Digital.pdf. (accessed October 22, 2021).
109. Huvepharma. Monimax
R . (2020). Available online at: https://www. huvepharma.com/news/article/unique-new-product-demonstratesefficacy-and-performance//. (accessed October 22, 2021).
110. Impextraco. Lerberk
R . (2019). Available online at: https://www.impextraco. com/products/enhancing-animals/xtra-performance-xp-anticoccidials/ coccidialsolution. (accessed October 22, 2021).
111. Feed additive Compendium. Narasin/Nicarbazin- Chickens (2018). p. 264.
112. Rose ME, Hesketh P. Immunity to coccidiosis: stages of the life-cycle of
Eimeria maxima which induce, and are affected by, the response of the host.
Parasitology. (1976) 73:25–37. doi: 10.1017/S0031182000051295
113. Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Exp Rev Vaccines. (2006) 5:143–63. doi: 10.1586/14760584.5.1.143
114. Rose ME, Wakelin D, Hesketh P. Eimeria vermiformis: differences in the course of primary infection can be correlated with lymphocyte responsiveness in the BALB/c and C57BL/6 mouse, Mus musculus. Exp
Parasitol. (1990) 71:276–83. doi: 10.1016/0014-4894(90)90032-8
115. Rose ME, Hesketh P, Rothwell L, Gramzinski RA. T-cell receptor gamma– delta lymphocytes and Eimeria vermiformis infection. Infect Immun. (1996)
64:4854–858. doi: 10.1128/iai.64.11.4854-4858.1996
116. Lillehoj HS, Choi KD. Recombinant chicken interferongamma- mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. (1998) 42:307–14. doi: 10.2307/1592481
117. Fatoba AJ, Adeleke MA. Diagnosis and control of chicken coccidiosis: a recent update. J Parasitic Dis. (2018) 42:483–93. doi: 10.1007/s12639-018-1048-1
118. Lillehoj HS. Effects of immunosuppression on avian coccidiosis: cyclosporin a but not hormonal bursectomy abrogates host protective immunity. Infect
Immun. (1987) 55:1616–21. doi: 10.1128/iai.55.7.1616-1621.1987
119. López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM. Overview of poultry eimeria life cycle and host-parasite interactions. Front Vet Sci. (2020)
7:384. doi: 10.3389/fvets.2020.00384
120. Shirley MW, Smith AL. Tomley FM. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasitol. (2005) 60:285–330. doi: 10.1016/S0065-308X(05)60005-X
121. Blake DP, Marugan-Hernandez V, Tomley FM. Spotlight on avian pathology:
Eimeria and the disease coccidiosis. Avian Pathol. (2021) 50:209–13. doi: 10.1080/03079457.2021.1912288
122. Blake DP, Pastor-Fernández I, Nolan MJ, Tomley FM. Recombinant anticoccidial vaccines-a cup half full?. Infect Genet Evol. (2017) 55:358–65. doi: 10.1016/j.meegid.2017.10.009
123. Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, et al.
Re-calculating the cost of coccidiosis in chickens. VetRes. (2020) 51:1–14. doi: 10.1186/s13567-020-00837-2
124. Jeffers TK. Attenuation of Eimeria tenella through selection for precociousness. J Parasitol. (1975) 75:1083–90. doi: 10.2307/3279381
125. Fetterer RH, Jenkins MC, Miska KB, Barfield RC. Evaluation of an experimental irradiated oocyst vaccine to protect broiler chicks against avian coccidiosis. Avian Dis. (2014) 58:391–7. doi: 10.1637/10679-092613-Reg.1
126. Gadelhaq SM, Arafa WM, Dahshan AHM, Abolhadid SM. Using of diclazuril in attenuation of Eimeria species for induction of protective immunity against coccidiosis in layer chicks. Assiut Vet Med J. (2017) 63:101–08. doi: 10.21608/avmj.2017.170969
127. Shirley MW. Bedrnik P. Live attenuated vaccines against avian coccidiosis: success with precocious and egg-adapted lines of Eimeria Parasitol. Today. (1997) 13:481–84. doi: 10.1016/S0169-4758(97)01153-8
128. Sharman PA, Smith NC, Wallach MG, Katrib M. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. (2010) 32:590–98. doi: 10.1111/j.1365-3024.2010.01209.x
129. Hoelzer K, Bielke L, Blake DP, Cox E, Cutting SM, Devriendt B, et al. Vaccines as alternatives to antibiotics for food producing animals.
Part 2: new approaches and potential solutions. Vet Res. (2018) 49:70. doi: 10.1186/s13567-018-0561-7
130. Pastor-Fernández I, Kim S, Marugán-Hernández V, Soutter F, Tomley FM,
Blake DP. Vaccination with transgenic Eimeria tenella expressing Eimeria maxima AMA1 and IMP1 confers partial protection against high-level E. maxima challenge in a broiler model of coccidiosis. Parasit Vect. (2020)
13:1–12. doi: 10.1186/s13071-020-04210-2
131. Sharaban H, Seadawy M, El-khayat F, El-Gohary AEG. Evaluation of coccidiosis vaccines in chicken. Kafr El-Sheikh Vet Med J. (2021) 19:13–19. doi: 10.21608/kvmj.2021.73171.1018
132. Kimminau EA. Duong T. Longitudinal response of commercial broiler operations to bio-shuttle administration. J Appl Poult Res. (2019) 28:1389–97. doi: 10.3382/japr/pfz092
133. Abbas RZ, Iqbal Z, Khan A, Sindhu ZUD, Khan JA, Khan MN, et al. Options for integrated strategie s for the control of avian coccidiosis. Int J Agric Biol. (2012) 14:1014–20
134. Cervantes HM. Antibiotic-free poultry production: is it sustainable? J Appl
Poult Res. (2015) 24:91–7. doi: 10.3382/japr/pfv006
135. Chapman HD, Barta JR, Blake D, Gruber A, Jenkins M, Smith NC, et al.
A selective review of advances in coccidiosis research. Adv Parasit. (2013)
83:93–171. doi: 10.1016/B978-0-12-407705-8.00002-1
136. Remmal A, Achahbar S, Bouddine L, Chami N, Chami F. In vitro destruction of Eimeria oocysts by essential oils. Vet Parasitol. (2011) 182:121–26. doi: 10.1016/j.vetpar.2011.06.002
137. Scheurer W, Spring P, Maertens L. Effect of 3 dietary phytogenic products on production performance and coccidiosis in challenged broiler chickens. J Appl Poult Res. (2013) 22:591–9. doi: 10.3382/japr.2013-
00726
138. Li J, Xing T, Wang L, Tao J. Liu, Z. Inhibitory effect of S-nitroso-glutathione on Eimeria tenella oocysts was mainly limited to the early stages of sporogony. Vet Parasitol. (2010) 173:64–9. doi: 10.1016/j.vetpar.2010.06.022
139. Rathinam T, Gadde U, Chapman HD. Sericea lespedeza has no anticoccidial effect when included in the diet of chickens infected with three species of
Eimeria. Vet Parasitol. (2014) 202:265–9. doi: 10.1016/j.vetpar.2014.01.017
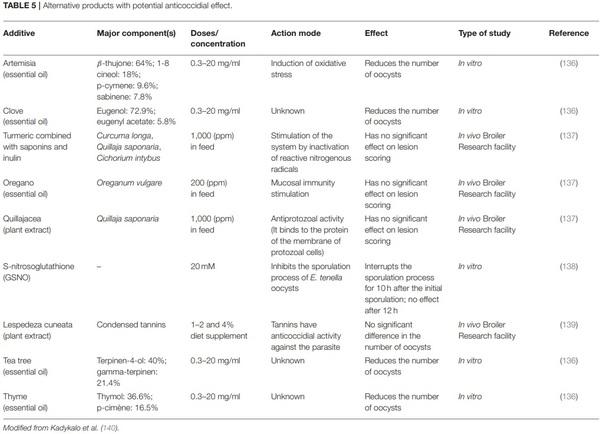
140. Kadykalo S, Roberts T, Thompson M, Wilson J, Lang M, Espeisse O.
The value of anticoccidials for sustainable global poultry production. Int J
Antimicrob Agents. (2018) 51:304–10. doi: 10.1016/j.ijantimicag.2017.09.004
141. Idris M, Abbas RZ, Masood S, Rehman T, Farooq U, Babar W, et al. The potential of antioxidant rich essential oils against avian coccidiosis. Worlds Poult Sci J. (2017) 73:89–104. doi: 10.1017/S00439339160
00787
142. Mesa C, Gómez-Osorio LM, López-Osorio S, Williams SM. ChaparroGutiérrez JJ. Survey of coccidia on commercial broiler farms in
Colombia: frequency of Eimeria species, anticoccidial sensitivity, and histopathology. Poult Sci J. (2021) 100:101239. doi: 10.1016/j.psj.2021.
101239
143. Mattiello R, Boviez JD. McDougald LR. Eimeria brunetti and
Eimeria necatrix in chickens of Argentina and confirmation of seven species of Eimeria. Avian Dis. (2000) 44:711–4. doi: 10.2307/159
3117
144. Prakashbabu BC, Thenmozhi V, Limon G, Kundu K, Kumar S, Garg R, et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet
Parasitol. (2017) 233:62–72. doi: 10.1016/j.vetpar.2016.12.003
145. Sun XM, Pang W, Jia T, Yan WC, He G, Hao LL, et al. Prevalence of Eimeria species in broilers with subclinical signs from fifty farms. Avian Dis. (2009)
53:301–5. doi: 10.1637/8379-061708-Resnote.1
146. Lee BH, Kim WH, Jeong J, Yoo J, Kwon YK, Jung BY, Min W. Prevalence and cross-immunity of Eimeria species on Korean chicken farms. J Vet Med Sci. (2010) 72:985–89. doi: 10.1292/jvms.09-0517
147. Gharekhani J, Sadeghi-Dehkordi Z. Bahrami M. Prevalence of coccidiosis in broiler chicken farms in Western Iran. J Vet Med. (2014) 2014:1–5. doi: 10.1155/2014/980604
148. Kaboudi K, Umar S, Munir MT. Prevalence of coccidiosis in freerange chicken in Sidi Thabet, Tunisia. Scientifica. (2016) 2016:1–6. doi: 10.1155/2016/7075195




United States









