1. Introduction.
There are limited data on the effectiveness of disinfectants against Lawsonia intracellularis, a Gram-negative obligately intracellular bacterium that causes proliferative enteropathy (PE) (Lawson and Gebhart, 2000). This is mainly due to the difficulty of finding good methods to measure the efficacy of disinfectants against an obligately intracellular bacterium. One study used a conventional tissue culture method to measure the viability status of L. intracellularis after exposure to some disinfectants (Collins et al., 2000). That assay was time-consuming and incapable of distinguishing between proportions of viable or nonviable bacteria. Recently, a modified tissue culture method that cultured bacteria in 96-well tissue culture plates has been developed for use in serological diagnosis (Guedes et al., 2002) and for the determination of the in vitro antimicrobial activity (Wattanaphansak et al., 2009) of L. intracellularis. This method allows the testing of a large number of compounds over various concentrations at the same time. Additionally, the viability of L. intracellularis in a mixed bacterial population can be directly measured using a direct count method with specific fluorescence staining (Wattanaphansak et al., 2005). Both assays make it possible to evaluate the effectiveness of disinfectants against L. intracellularis using bacterial viability as an indicator.
Stalosan1 F (Stormollen, Tureby, Storstrom, Denmark), a powder disinfectant mainly composed of phosphate compounds (85%), copper sulfate (2.5%), ferrous sulfate (2.1%), active chlorine (0.25%), perica oil (0.05%), and alsilicate (10.1%), is indicated for use in livestock farms for reducing the number of microorganisms in the environment, absorption of moisture, and reduction of ammonia production. The susceptibility of L. intracellularis to Stalosan F has not been reported. Therefore, the objective of this study was to evaluate both the modified tissue culture method and the direct count method for testing the efficacy of disinfectants against L. intracellularis and to determine the bacteriocidal activity of Stalosan F against L. intracellularis using both methods.
2. Materials and methods.
2.1. Microorganism strains and preparations.
L. intracellularis strains VPB4 and PHE/MN-01, which were isolated from proliferative hemorrhagic enteropathy infected pigs in the United States in 1991 and 2000, respectively (Guedes and Gebhart, 2003a), were used throughout this study, prepared independently and tested twice. Both were grown, maintained, and harvested as described previously (Guedes and Gebhart, 2003b; Wattanaphansak et al., 2005). The final concentration of L. intracellularis was determined using a direct count staining procedure as described previously (Guedes and Gebhart, 2003b).
2.2. Disinfectants and test procedures.
Two forms of Stalosan F preparations, a powder disinfectant and an aqueous suspension, were used for testing. For use as a powder, Stalosan F was tested at three final concentrations which were 2X, 1X, and 0.5X of recommended dosages (X = dose recommended on label). Three hundred microliters of bacterial solution containing approximately 108 L. intracellularis/ml were added in duplicate and spread onto 10 cm 10 cm square sterile Petri dishes. Then, 1 g, 0.5 g, or 0.25 g of Stalosan F powder was applied evenly to cover the entire surface of the Petri dish. These yielded final concentrations of Stalosan F equivalent to 100 g/m2 , 50 g/m2 , and 25 g/m2 , respectively.
For testing as an aqueous suspension, Stalosan F was prepared to final concentrations of 1%, 4%, 8%, 16%, and 32% in Dulbecco’s modified Eagle medium (DMEM). The suspension was mixed and 8 ml of each concentration was aliquoted in duplicate, with the addition of 300 ml of 108 L. intracellularis/ml to each.
In both applications, L. intracellularis was exposed to Stalosan F for 0.5 h, 1 h, 2 h, and 4 h at room temperature. The controls for each time point were live L. intracellularis in DMEM without exposure to Stalosan F and dead L. intracellularis in which the bacteria were exposed to isopropyl alcohol for 30 min. After incubation, the powder in the Petri dishes was washed with 8 ml DMEM and the suspension was immediately transferred to 15 ml tubes. The bacteria in both applications were separated from the powder by passing the suspension through 5 mm filters into microcentrifuge tubes and centrifuged at 10,000 rpm for 3 min. The pellet was washed twice with sterile distilled water and half was re-suspended with 2 ml sterile distilled water for enumeration by the direct count method. The other half was re-suspended with 2 ml of L. intracellularis culture media and used to infect 1-day-old McCoy cells in the modified tissue culture method.
2.3. Bacterial survival assay.
The percentage of L. intracellularis surviving after exposure to the disinfectant was assessed using both direct count and the modified tissue methods. The direct count method was conducted using Live/Dead1 BacLightTM staining as described in a previous study (Wattanaphansak et al., 2005). In this study, only green fluorescent cells of live bacteria that stained with SYTO-9 were counted. The modified tissue culture method was performed as described previously (Wattanaphansak et al., 2009) to determine L. intracellularis viability. The effectiveness of Stalosan F was determined by evaluating the number of heavily infected cells (HICs), which were defined as the relative number of cells that were infected with the surviving L. intracellularis after exposure to the Stalosan F. These numbers were used as a live L. intracellularis indicator.
2.4. Scanning electron microscopy (SEM).
For SEM observations, L. intracellularis was exposed to 0.5 g of Stalosan F powder and 16% of Stalosan F suspension for 30 min. The bacteria were then filtered through a 5 mm filter and washed with phosphate buffer saline (PBS) twice. The samples were fixed with 2.5% glutaraldehyde in PBS for 1 h at room temperature. After three washes with PBS, the bacterial cells were fixed with 1% osmium tetroxide in PBS and washed three more times with PBS. The bacterial cells were dehydrated with increasing concentrations of ethanol (25%, 50%, 75%, 95%, and 100%) and dried in a Balzer Critical Point Dryer 0101 unit. The fixed bacteria were coated with a thin film of gold–palladium and examined using a VPSEM-Hitachi S-3500N scanning electron microscope.
2.5. Data analysis.
The number of HIC and the number of green fluorescent bacteria in each treatment of Stalosan F were expressed as percentages as compared to the controls. The correlation between the modified tissue culture and direct count methods was estimated with Spearman’s coefficient of rank correlation using the MedCalc1 version 9.1.0.1 software.
3. Results.
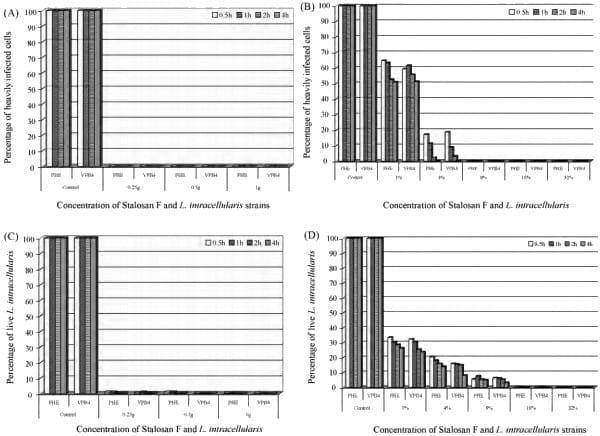
The results (Figs. 1 and 2) show that both strains of L. intracellularis were similar in their susceptibilities to both powder and aqueous suspensions of Stalosan F. The modified tissue culture method was used to determine the surviving population and most of the McCoy cells in live control wells were heavily infected with L. intracellularis, indicating that the percentage of L. intracellularis infection was close to 100%. In contrast, no HICs were detected after exposure to 0.25 g/cm2 , 0.5 g/cm2 , and 1 g/ cm2 of Stalosan F for 30 min, indicating 100% inactivation compared to the live control (Figs. 1A and 2B).
Fig. 1. The effectiveness of Stalosan F used as a powder (A) and as an aqueous suspension (B) against Lawsonia intracellularis measured with the modified tissue culture method. The effectiveness of Stalosan F used as a powder (C) and as an aqueous suspension (D) against L. intracellularis measured with the direct count method.
When Stalosan F was tested as an aqueous suspension, the number of HICs decreased with increased concentrations of Stalosan F and increased exposure time. The viability of L. intracellularis decreased to approximately 65% after exposure to 1% of Stalosan F concentration for 30 min. The surviving population of L. intracellularis was markedly reduced in the aqueous concentration of 4% and no L. intracellularis was detected in the tissue culture at a concentration of 8% for 30 min (Fig. 1B). Under these conditions, both the powder and suspension forms of Stalosan F have a bactericidal effect on L. intracellularis.
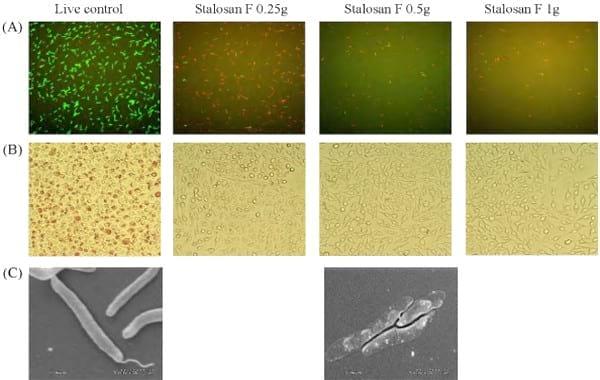
When using the direct count method to determine L. intracellularis viability, approximately 95% of live control bacteria exhibited a green fluorescence of SYTO-9, indicating live bacteria with an intact cell membrane. In contrast, a small percentage of mixed bacterial populations had a red fluorescence of propidium iodine, indicating dead bacteria with a damaged cell membrane (Fig. 2A). After 30 min of exposure to Stalosan F powder, the number of live bacteria progressively decreased to less than 1% in all tested concentrations of powder Stalosan F (Fig. 1C). In contrast, more red fluorescent bacteria were observed, indicating that the bacterial structure might be damaged or lysed after contact with Stalosan F.
Similar results were found when L. intracellularis was exposed to an aqueous suspension of Stalosan F. The viable populations of L. intracellularis decreased with increased concentrations of Stalosan F in the suspension. The number of live bacteria was approximately 30% after exposure to 1% of Stalosan F for 30 min and less than 1% in concentrations of 16%, with more than 99% of L. intracellularis killed when compared to the controls (Fig. 1D).
In this study, the results obtained from the modified tissue culture method were similar to the results obtained from the direct count method. There was a significantly positive correlation between both assays (r = 0.76, p = 0.001) indicating a good agreement between both methods.
The morphological appearance of L. intracellularis after 30 min of exposure to Stalosan F is shown in Fig. 2C. Electron microscopy showed the presence of flagellar components on untreated L. intracellularis cells and the cell wall of the bacterium seemed cloudy and intact. After exposure to Stalosan F powder at 0.5 g for 30 min, the bacterial cell wall became more translucent, indicating damage to the cell wall. The similar appearance of bacteria is found after exposure to aqueous suspension at 4% and 16% of Stalosan F (picture not shown).
Fig. 2. L. intracellularis exposed to Stalosan F powder at 0.25 g, 0.5 g, and 1 g for 0.5 h. The viability of L. intracellularis was measured with the direct count method (A) and the modified tissue culture method (B). Scanning electron micrographs of normal L. intracellularis and after exposure to 0.5 g of Stalosan F (C).
4. Discussion.
The use of chemical disinfectants in swine facilities is a first line of defense against virus, bacteria, and parasite infection. However, the effect of disinfectants on L. intracellularis is very difficult to measure in vitro. Unlike other bacteria, L. intracellularis is an organism that propagates itself only inside enterocytes. Cell-free culture methods have not been successfully established and so there are no standard in vitro assays for assessing the efficacy of disinfectants against L. intracellularis. In this study, we compared two systems, a modified tissue culture method and a direct count method, for evaluating the efficacy of Stalosan F in killing L. intracellularis.
The results from both methods showed that either the powder or suspension forms of Stalosan F could be used for L. intracellularis inactivation. The reduction of L. intracellularis viability depended on dose and exposure time of Stalosan F. According to the manufacturer, the compound is to be applied directly on the floor at a concentration of 50 g/m2 , which equaled to 0.5 g/cm2 in this study, for reducing the number of viable organisms in the environment. This concentration would be able to kill 100% or > 99% of L. intracellularis after exposure for 30 min, according to the tissue culture and direct count assay, respectively. Although the mode of action of Stalosan F is not fully understood, it has been shown that the viabilities of pathogenic bacteria and viruses were significantly reduced when those organisms were exposed to Stalosan F (Methling et al., 1997).
After exposure to the powder and suspension forms of Stalosan F, a few viable L. intracellularis (Stalosan F remains unclear, it is possible that the membrane of the bacteria was destroyed by Stalosan F. The abnormality of L. intracellularis membranes was observed using the SEM, as some parts of the bacterial membrane were clear and transparent after treatment.
5. Conclusion.
In summary, we demonstrated that the modified tissue culture and the direct count methods gave similar results for measuring the viability status of L. intracellularis after exposure to a powder disinfectant. Our results indicate that Stalosan F in both a powder concentration of 0.25 g/ cm2 and an aqueous suspension of 16% concentration are able to inactivate over 99% of both L. intracellularis strains after 30 min of exposure as determined by both assays. Acknowledgements This study was supported in part by a grant from Phibro Animal Health. The authors would like to thank Benjawan Wijarn and Molly Freese for excellent technical assistance.
References.
1. Collins, A.M., Love, R.J., Pozo, J., Smith, S.H., McOrist, S., 2000. Studies on the ex vivo survival of Lawsonia intracellularis. Swine Health Prod. 8, 211–215.
2. Guedes, R.M.C., Gebhart, C.J., Deen, J., Winkelman, N.L., 2002. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J. Vet. Diag. Invest. 14, 528–530.
3. Guedes, R.M.C., Gebhart, C.J., 2003a. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diag. Invest. 15, 438–446.
4. Guedes, R.M.C., Gebhart, C.J., 2003b. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet. Microbiol. 91, 135–145.
5. Gupte, A.R., De Rezende, C.L., Joseph, S.W., 2003. Induction and resuscitation of viable but nonculturable Salmonella enterica serovar typhimurium DT104. Appl. Environ. Microbiol. 69, 6669–6675.
6. Lawson, G.H.K., Gebhart, C.J., 2000. Proliferative enteropathy. J. Comp. Pathol. 122, 77–100.
7. Methling, W., Dibbert, R., Heinrich, H.W., 1997. Stalosan F- not a chemical disinfectant, but a very good hygiene substance. In: Proceedings of 9th International Congress in Animal Hygiene, Finland, p. 387.
8. Millard, P., Roth, B., 1997. Fluorescence-based methods for microbial characterization and viability assessment. Biotechnol. Int. 1, 291– 296.
9. Roszak, D.B., Grimes, D.J., Colwell, R.R., 1984. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can. J. Microbiol. 30, 334–338.
10. Tholozan, J.L., Cappelier, J.M., Tissier, J.P., Delattre, G., Federighi, M., 1999. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65, 1110–1116.
11. Wattanaphansak, S., Gebhart, C.J., Olin, M., Deen, J., 2005. Measurement of the viability of Lawsonia intracellularis. Can. J. Vet. Res. 69, 265– 271.
12. Wattanaphansak, S., Singer, R.S., Gebhart, C.J., 2009. In vitro antimicrobial activity against 10 North American and European Lawsonia intracellularis isolates. Vet. Microbiol. 134, 305–310.
13. Xu, H.S., Roberts, N., Singleton, F.L., Attwell, R.W., Grimes, D.J., Colwell, R.R., 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8, 313–323.