Direct and indirect interactions between Eastern Tent Caterpillars and mare reproductive loss syndrome
Published: March 29, 2007
By: BRUCE A. WEBB - University of Kentucky (Courtesy of Alltech Inc.)
In April and May of 2001 an epidemic of equine abortions struck in the Ohio River valley that induced a loss of about 30% in 2002 in central Kentucky (Kane and Kriby, 2001; Veterinary Sciences, 2002).
Through mid-April, equine abortions were not elevated over prior years but unusual early and late term abortions were noted in late April and continued through much of May. Coincident with these losses some animals were also afflicted with uveitis and pericarditis, albeit at much lower frequencies (Veterinary Sciences, 2002). Vigorous investigations into this phenomenon, subsequently termed ‘mare reproductive loss syndrome’ (MRLS), were initiated that ultimately associated the syndrome with unusual weather patterns and elevated populations of Eastern Tent Caterpillars (ETC). Anecdotal and survey data documented that a freeze on April 17 and 18 that followed an unusually warm and dry early April preceded the onset of the syndrome.
The abortions were subsequently correlated with the presence of ETC larvae in or around affected pastures and, to a lesser degree, with the presence of wild cherry trees. These findings led to formulation of the hypothesis in late May that MRLS was caused by cyanide delivered to the horses through a direct or indirect interaction with Eastern Tent Caterpillars (Veterinary Sciences, 2002).
This hypothesis was based on the following points: (1) black cherry trees (Prunus serotina) line many horse pastures in central Kentucky, (2) cherry leaves have the capacity to produce cyanide through degradation of cyanogenic plant precursors with cyanogenic capacity of leaves highest in spring (Smeather et al., 1973), (3) ETC larvae preferentially feed on cherry trees (Peterson et al., 1987; Schroeder, 1978) and were abundant in central Kentucky in 2001, (4) cyanide was detected in foreguts of ETC larvae (Fitzgerald et al., 2001; Tobin et al., personal communication; Webb and Collins, unpublished), (5) the presence of cherry trees and ETC larvae was correlated with incidence of MRLS (Veterinary Sciences, 2002) and (6) some aborted fetuses and mares were found to have elevated levels of cyanide in some tissues (Veterinary Sciences, 2002).
Although consistent with much of the information available at the time proposed, there are weaknesses in the cyanide hypothesis. For example, cyanide is noted for its direct toxicity on oxygen transport with cyanide poisoning usually associated with some level of fatality (Faust, 1994; Vetter, 2000). Although incidence of MRLS was widespread, there were no reports of fatalities other than foals associated with MRLS in 2001. The reproductive effects of sub-lethal cyanide doses in animal systems are not as well documented as other toxicological effects. Given that cyanogenic plants are ubiquitous (Smeather et al., 1973; Vetter, 2000) and detoxification mechanisms in animals have been studied in some depth (Meyers and Ahmad, 1991), if abortions were frequently associated with cyanide exposure one would expect it to be well-documented in the literature. However, there are few reports that link exposure to sub-lethal levels of cyanide with widespread abortions in mammals and essentially no information on such an association in horses.
From an entomological perspective Eastern Tent Caterpillars seem unlikely to deliver cyanide to horses as efficiently as plant material. Insects that feed on cyanogenic plants have the capacity to metabolize the compound (Meyers and Ahmad, 1991), probably by conjugating to alanine to form cyanoalanine, with this compound subsequently metabolized to the non-toxic amino acid arginine. When the detoxification capacity of lepidopteran larvae that normally feed on cyanogenic plants was deliberately exceeded in laboratory studies by feeding an artificial diet containing 1.5 % cyanide, the larvae were susceptible to the toxin and were transiently paralyzed (Brattsten et al., 1983).
However, these studies were not performed on Eastern Tent Caterpillars because of their highly seasonal availability. In the spring of 2001, the literature had no publications specifically addressing the detoxification, abundance or metabolism of cyanogenic compounds by Eastern Tent Caterpillars.
In addition, herbivorous insects are known to metabolize cyanogenic plant compounds through different pathways (Woodward and Bernays, 1977), making it difficult to assess the cyanide hypothesis without additional study. ETC feed preferentially on one of the most highly cyanogenic plants known, Prunus serotina or black cherry (Smeather et al., 1973; Vetter, 2000). The data did not eliminate the possibility that ETC larvae sequester cyanide or had specialized capabilities that allowed substantial quantities of cyanide to pass through the insect gut.
Thus additional study was required to address the possibility that cyanide was vectored by ETC to horses and thereby contributed to MRLS.
The Eastern Tent Caterpillar
Eastern Tent Caterpillars are a ubiquitous and abundant insect over most of the eastern United States (Figure 1). Because of their tent-building habit, these insects are particularly noticeable and have been recognized as defoliators of trees during outbreak years since colonial times. There are few insects having greater direct contact with humans and they are generally considered innocuous (Schroeder, 1978), even appropriate subjects for study in elementary school classrooms. Although outbreaks of tent caterpillars reportedly stopped trains in New England due to the lubricating effects of their carcasses on the tracks and entomologists have organized communities to manually remove tent caterpillar egg masses and nests to reduce local populations, ETC larvae have not been associated with significant vertebrate toxicity. Allergic symptoms have sometimes been associated with ETC larvae and include reactions to larval setae (hairs) and blood (hemolymph), usually among entomologists working intensively with the insects (Fitzgerald, 1995).

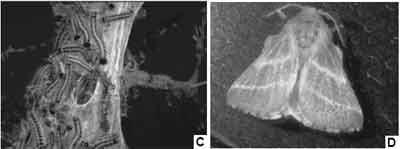
Figure 1. Eastern Tent Caterpillar developmental stages.
A. Eastern Tent Caterpillar egg mass on cherry branch.
B. Eastern tent caterpillar larvae on tent surface.
C. Eastern Tent Caterpillar larvae and trail webbing deposited as larvae leave defoliated tree in search of alternative food sources.
D. Eastern Tent Caterpillar adult moth.
Over recent decades, control of tent caterpillars has been recommended only for aesthetic purposes as early spring defoliation has little lasting effect on productivity and survival of most trees (Bessin, 1995). The negative impact on the environment associated with controlling ETC populations with insecticides and the cost were considered more significant than the seasonal and episodic nuisance of occasional early spring defoliation. ETC populations harbor a number of diseases that reach epidemic proportions as populations rise and typically cause the population to crash after a few outbreak years. Thus, if their aesthetic impact can be tolerated, ETC outbreaks are effectively transient and self-limiting.
Because anecdotal and survey data support a correlation between ETC, black cherry trees and MRLS and little support was developed for other hypotheses, researchers focused on evaluating the cyanide hypothesis from both entomological and veterinary perspectives. Results from veterinary studies are not considered here as my laboratory has only evaluated the cyanide hypothesis from an entomological perspective. Specifically, we sought to address the following issues.
(1) Do ETC larvae concentrate cyanide in insect tissues? This possibility was addressed by measuring cyanide levels in cherry leaves and in feeding larvae.
(2) Does cyanide pass through ETC larvae so that it might be delivered in quantities sufficient to intoxicate horses via ingestion of forage contaminated with insect excrement (frass)? This possibility was addressed by measuring cyanide and cyanogenic plant precursors in the insect gut and in frass.
(3) Do ETC larvae release cyanide into water? It was suggested that larvae in pools and buckets may have delivered cyanide to horses.
(4) What effect does starvation have on cyanide levels in ETC? The spring of 2001 was unusual in that their preferred host trees were defoliated well before ETC larvae completed their development. This forced larvae into pastures in search of alternative food sources.
(5) Do cyanide levels change over the course of ETC development in such a way as to facilitate delivery of the toxin to horses? As described below, the results of studies conducted over the summer of 2001 suggest that ETC larvae are unlikely to deliver significant quantities of cyanide to horses.
Of necessity, our studies were conducted with ETC larvae shipped to us from areas north of Kentucky and fed on local black cherry foliage collected in June. The possibility that our results would differ if conducted earlier in the year cannot be eliminated, although the similar findings of Fitzgerald et al. (2001) make this unlikely.
Detection of cyanide in Eastern Tent Caterpillars
To evaluate the concentration of cyanide in ETC larvae, insects from Maine and Vermont were fed black cherry leaves from central Kentucky in early June. Two assays were established using potassium cyanide (KCN) and the plant cyanogenic glycoside, prunasin, to standardize the assay. The first assay was based on sodium picrate paper test. The sodium picrate assay has been used for approximately 100 years to detect gaseous cyanide from a number of sources, including homogenates of plant or insect tissues (Dahlman and Johnson, 1980; Brinker and Seigler, 1989). This assay is recognized to be robust but not as sensitive as other methods and not strictly quantifiable. Insects were dissected to investigate the levels of cyanide in the three major regions of the lepidopteran gut, the foregut, midgut and hindgut with the rest of the insect also homogenized and assayed (carcass).
In this initial test, cyanide was readily detected in leaves and in the insect foregut but was almost undetectable in the midgut, hindgut, frass and carcass. Little digestion occurs in insect foreguts (crop), and the ingested plant material in these feeding ETC larvae was visibly green. These results are consistent with those reported by Fitzgerald et al. (2001), who measured cyanide levels of black cherry leaf tissue averaging 1,131 and 3,032 ppm in stems and fresh leaves respectively, an average of 631 ppm in foreguts and 14 ppm in the midgut. Similarly, cyanide levels in frass ranged from 20 to 85 ppm in fresh and dried frass, respectively. By way of comparison, mammalian toxic doses, in terms of LD50, range from 1 to 7 mg/kg body weight (Faust, 1994).
In plants, cyanide is stored as cyanogenic glycosides with enzymes released when the tissue is crushed or ingested that break down the cyanogenic glycosides to release cyanide. The release of cyanide is thought to protect the plants from generalist herbivory (Woodward and Bernays, 1977), but many insects are known to specialize on and prefer cyanogenic plants (Brattsten et al., 1983). Black cherry trees (Prunus serotina) store cyanide as prunasin (β-D-mandelonitrile-glucoside) with a β-glucosidase released to cleave the sugar moiety from prunasin to form the intermediate molecule mandelonitrile (Figure 2). Mandelonitrile spontaneously decays under many physiological conditions to release cyanide and benzaldehyde.
Interestingly, ETC larvae are known to regurgitate the partially digested leaves from the foregut as a defensive reaction when attacked by ants (Peterson et al., 1987). Bioassays of the reactions of ants to cyanide and benzaldehyde suggest that only the latter is a deterrent to ant predation. Because cyanogenic glycosides are precursors for cyanide production, assessing the cyanide that could be produced requires the measuring of cyanide, mandelonitrile and prunasin to determine the total cyanogenic capacity. Conventionally, the plant precursors are converted to cyanide through addition of exogenous β-glucosidase with only the cyanide moiety directly quantified. We adopted and standardized the assay of Esser et al. (1993) with cyanide and β-D-mandelonitrile glucoside for this purpose. Conversion of prunasin to cyanide was near quantitative on a molar basis in standard curves and in test assays with known amounts of cyanide or prunasin in homegenates of insect tissues.
Quantification of preliminary results using the Esser et al. (1993) assay supported our preliminary findings and those of Fitzgerald et al. (2001) in showing that cyanide was found in quantity only in the foregut of feeding larvae. Furthermore, conversion assays to detect prunasin and mandelonitrile demonstrated that the cyanogenic precursors were metabolized in the foregut with negligible quantities detected in the larval midgut or hindgut (Webb and Collins, unpublished). I have estimated the amount of cyanide potentially vectored by Eastern Tent Caterpillars relative to that available through ingestion of plant material. Insect foreguts have about 33% of the cyanogenic capacity of plant tissue on a weight basis. The foregut is about 15% of the total body weight of a feeding larvae, so ETC larvae have about 5% of the cyanide concentration of leaves. ETC frass had about 1% or less of the cyanide levels found in black cherry leaves. In other words, a horse would have to ingest 20 times more larvae and 100 times more frass than black cherry leaves to accumulate an equivalent dose of cyanide. These results indicate that the potential for ETC to vector cyanide to other animals, including horses, is significantly less than that potentially delivered through direct ingestion of plant material.
We have gone on to consider some other factors. We have starved larvae and determined that by 6 hrs of starvation, 9 of 10 larvae had emptied their foreguts of food and cyanide. We assayed feeding larvae for their ability to release cyanide into water and found that submerging larvae of ~1 g in 50 ml of water did not result in the release of cyanide at levels detectable in our assays. Similarly, larvae in the physiological state known as ‘wandering’, in which they search for a pupation site, had no detectable levels of cyanide. These wandering larvae are most noticeable on the ground and most likely to come into contact with horses. Taken together, these studies suggest that only larvae which have been feeding recently on leaves could vector cyanide and further support the conclusion that ETC are unlikely to vector significant quantities of cyanide to horses.
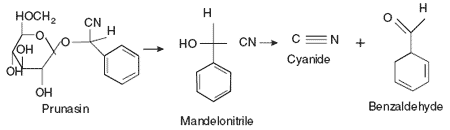
Figure 2.Breakdown of prunasin to release cyanide. The plant cyanogenic glycoside, prunasin, is digested byß-glucosidases to produce mandelonitrile (and glucose). Under neutral and basic conditions, mandelonitrile decays to produce benzaldehyde and the cyanide ion.
There is a possibility that ETC larvae are toxic through some other mechanism that is unrelated to cyanide levels. Again, the data are not available in which ETC larvae have been fed to horses to assess this possibility. However, there is at least one study in which ETC larvae were dried, homogenized and used as a dietary source of protein for rodents. In this study, dried ETC homogenates were fed to rats at concentrations up to 61.5% of dietary nitrogen (Finke et al., 1989). At the two highest doses, diarrhea symptoms were observed and dissection revealed intestinal abnormalities that were suggestive of toxicity. The tent caterpillar meal also seemed less palatable than other insect meals. However, it seems unlikely that horses would have ingested ETC larvae in quantities that were required to induce toxicity in rodents or in anything approaching the levels of dietary nitrogen used in the rodent study.
Other theories related to Eastern Tent Caterpillars As the likelihood that ETC larvae had delivered cyanide to horses decreased, other hypotheses were developed that may explain the relationship between MRLS and ETC. These are briefly described below.
NUTRIENT FLOW OR ‘FRASS-FUNGUS’ HYPOTHESIS
This hypothesis, developed in concert with Dr. Newman of Venture Labs, posits that frass deposited by ETC larvae onto pastures promoted growth of some fungi during early April. The freeze of April 17 and 18 then acted upon the fungi to induce mycotoxin production. When frass-contaminated forage was ingested by horses, the mycotoxins produced by the fungi in the frass could be the proximate cause of MRLS.
DISEASE VECTOR HYPOTHESIS
The disease vector hypothesis posits that a nonconventional infectious agent was transmitted from ETC larvae to horses. There are many insects that transmit disease, most notably those such as mosquitoes and biting flies that are blood-feeders.
Other insects, notably cockroaches and houseflies, transmit diseases through contamination of foods and surfaces. An infectious cause of MRLS was ruled out, in part because of the simultaneous onset of the disease across a wide area. While most infectious diseases do not have this etiology, the appearance of insect-vectored diseases is closely associated with temporal occurrence and exposure to the insect vector. It follows that it might be possible for insects to vector a disease almost simultaneously across a widespread area if the occurrence of the insect is transient. The outbreak of ETC larvae in 2001 provides some of the conditions consistent with the disease vector hypothesis.
IMMUNE SUPPRESSION HYPOTHESIS
Lepidopteran larvae are known to cause allergic reactions among entomologists, which can sometimes be severe. The most common toxicity associated with ETC larvae is a mild irritation of the skin. There is some evidence that the cellular immune system of mares was suppressed (Harrison, personal communication) suggesting that the syndrome involved an insult to the immune system.
This is also consistent with the opportunistic occurrence of bacteria in the placenta of early foals (40-100 days gestation) lost to the syndrome. Thus it seems possible that exposure to ETC larvae may have directly or indirectly affected the immune systems of mares and contributed to MRLS.
ESTROGEN/ANTIESTROGEN HYPOTHESIS
In preliminary analyses of estrogenic and antiestrogenic acitvity, ETC larvae, frass and cherry leaves had significant amounts of anti-estrogenic activity (K. McDowell, personal communication). Based on these data Dr. McDowell has formulated the hypothesis that antiestrogens associated with ETC or cherry leaves may contribute to MRLS. Antiestrogens are known to be capable of inducing abortions.
Although some data support each of the hypotheses described above, it is not possible to evaluate them critically or to focus on one as most likely because all lack critical data. Therefore, my laboratory and colleagues in this work have chosen to focus on the more fundamental question of whether ETC larvae contributed to MRLS. In this way, we make no assumptions as to the mechanism through which ETC may be involved, but simply ask the question of whether they are involved and take measures during the experiment that may lead us to focus on one or more specific hypotheses in subsequent trials and experiments.
Manipulation of direct and indirect exposure to ETC
In the spring of 2002, the association between ETC and MRLS will be investigated with some efforts to mimic some environmental conditions that were suspected to contribute. Rather than design experiments to test each hypothesis, we plan to manipulate the level of exposure of mares in a susceptible stage of pregnancy to ETC larvae and/ or their excrement. We plan two experimental treatments and a control with the experiment designed such that induction of MRLS at levels seen in 2001 would produce a statistically significant result in a single trial. We plan two trials with attempts to mimic the environmental insult thought to be linked to MRLS (the hard freeze) during the second trial.
In the direct exposure to ETC trial, we plan to expose mares between 40 and 100 days gestation to a minimum of 20,000 larvae in small pens for 6 hrs/day over a 10 day period in two separate trials. In the indirect exposure trial, we plan to seed plots with frass from the same number of larvae over the same time period. A control group is included that will receive identical handling but no insect treatments. The hard freeze will be mimicked by chilling frass and/or insects to -2o C overnight prior to delivery to the experimental plots.
It is impossible to replicate all of the environmental conditions, but this experiment has been developed in an attempt to address those two factors, ETC and temperature, that are thought to be involved.
MRLS in a historical context
In 1980 and 1981, phenomena similar to MRLS were reported although records suggest that the severity of the syndrome was less than that experienced in 2001. These years were also notable for their unusual spring weather and high populations of ETC. If a similar causative agent(s) contributed to the equine abortions of 1980 and 1981, then one must assume that the syndrome has been episodic, but unrecognized, for some time, perhaps over 200 years. If so, MRLS did not prevent the establishment and flourishing of the equine industry in central Kentucky and the historical record suggests that future episodes will be infrequent and have a modest impact on the industry over the long term.
Of course, as events of 2001 attest, some years may be very difficult. The prospect that MRLS may be correlated with identified environmental conditions such as ETC population levels and spring weather conditions opens the potential for the equine industry to take preventative measures even in the absence of a known cause. This is the rationale for establishing the MRLS Environmental Monitoring Program at the University of Kentucky. If factors correlated with the occurrence of a disease can be identified, it is possible to control the spread of disease even without knowledge of the etiological agent.
A major complication in study of MRLS is its episodic nature. One can simply not expect to have an occurrence every year and this is likely to make it particularly difficult to investigate. The limited historical record indicates that MRLS is rare, occurring once or twice every 20 years. A human disease that is in some ways similar is the hemorrhagic fever caused by the Ebola virus.
Although the etiological agent causing this disease has been identified (unlike MRLS), its reservoir and sylvatic mode of transmission is not known, principally because of its episodic and unpredictable occurrence. These circumstances suggest that understanding MRLS may require a sustained effort to elucidate its causes and eliminate its impact on the equine industry.
References
Through mid-April, equine abortions were not elevated over prior years but unusual early and late term abortions were noted in late April and continued through much of May. Coincident with these losses some animals were also afflicted with uveitis and pericarditis, albeit at much lower frequencies (Veterinary Sciences, 2002). Vigorous investigations into this phenomenon, subsequently termed ‘mare reproductive loss syndrome’ (MRLS), were initiated that ultimately associated the syndrome with unusual weather patterns and elevated populations of Eastern Tent Caterpillars (ETC). Anecdotal and survey data documented that a freeze on April 17 and 18 that followed an unusually warm and dry early April preceded the onset of the syndrome.
The abortions were subsequently correlated with the presence of ETC larvae in or around affected pastures and, to a lesser degree, with the presence of wild cherry trees. These findings led to formulation of the hypothesis in late May that MRLS was caused by cyanide delivered to the horses through a direct or indirect interaction with Eastern Tent Caterpillars (Veterinary Sciences, 2002).
This hypothesis was based on the following points: (1) black cherry trees (Prunus serotina) line many horse pastures in central Kentucky, (2) cherry leaves have the capacity to produce cyanide through degradation of cyanogenic plant precursors with cyanogenic capacity of leaves highest in spring (Smeather et al., 1973), (3) ETC larvae preferentially feed on cherry trees (Peterson et al., 1987; Schroeder, 1978) and were abundant in central Kentucky in 2001, (4) cyanide was detected in foreguts of ETC larvae (Fitzgerald et al., 2001; Tobin et al., personal communication; Webb and Collins, unpublished), (5) the presence of cherry trees and ETC larvae was correlated with incidence of MRLS (Veterinary Sciences, 2002) and (6) some aborted fetuses and mares were found to have elevated levels of cyanide in some tissues (Veterinary Sciences, 2002).
Although consistent with much of the information available at the time proposed, there are weaknesses in the cyanide hypothesis. For example, cyanide is noted for its direct toxicity on oxygen transport with cyanide poisoning usually associated with some level of fatality (Faust, 1994; Vetter, 2000). Although incidence of MRLS was widespread, there were no reports of fatalities other than foals associated with MRLS in 2001. The reproductive effects of sub-lethal cyanide doses in animal systems are not as well documented as other toxicological effects. Given that cyanogenic plants are ubiquitous (Smeather et al., 1973; Vetter, 2000) and detoxification mechanisms in animals have been studied in some depth (Meyers and Ahmad, 1991), if abortions were frequently associated with cyanide exposure one would expect it to be well-documented in the literature. However, there are few reports that link exposure to sub-lethal levels of cyanide with widespread abortions in mammals and essentially no information on such an association in horses.
From an entomological perspective Eastern Tent Caterpillars seem unlikely to deliver cyanide to horses as efficiently as plant material. Insects that feed on cyanogenic plants have the capacity to metabolize the compound (Meyers and Ahmad, 1991), probably by conjugating to alanine to form cyanoalanine, with this compound subsequently metabolized to the non-toxic amino acid arginine. When the detoxification capacity of lepidopteran larvae that normally feed on cyanogenic plants was deliberately exceeded in laboratory studies by feeding an artificial diet containing 1.5 % cyanide, the larvae were susceptible to the toxin and were transiently paralyzed (Brattsten et al., 1983).
However, these studies were not performed on Eastern Tent Caterpillars because of their highly seasonal availability. In the spring of 2001, the literature had no publications specifically addressing the detoxification, abundance or metabolism of cyanogenic compounds by Eastern Tent Caterpillars.
In addition, herbivorous insects are known to metabolize cyanogenic plant compounds through different pathways (Woodward and Bernays, 1977), making it difficult to assess the cyanide hypothesis without additional study. ETC feed preferentially on one of the most highly cyanogenic plants known, Prunus serotina or black cherry (Smeather et al., 1973; Vetter, 2000). The data did not eliminate the possibility that ETC larvae sequester cyanide or had specialized capabilities that allowed substantial quantities of cyanide to pass through the insect gut.
Thus additional study was required to address the possibility that cyanide was vectored by ETC to horses and thereby contributed to MRLS.
The Eastern Tent Caterpillar
Eastern Tent Caterpillars are a ubiquitous and abundant insect over most of the eastern United States (Figure 1). Because of their tent-building habit, these insects are particularly noticeable and have been recognized as defoliators of trees during outbreak years since colonial times. There are few insects having greater direct contact with humans and they are generally considered innocuous (Schroeder, 1978), even appropriate subjects for study in elementary school classrooms. Although outbreaks of tent caterpillars reportedly stopped trains in New England due to the lubricating effects of their carcasses on the tracks and entomologists have organized communities to manually remove tent caterpillar egg masses and nests to reduce local populations, ETC larvae have not been associated with significant vertebrate toxicity. Allergic symptoms have sometimes been associated with ETC larvae and include reactions to larval setae (hairs) and blood (hemolymph), usually among entomologists working intensively with the insects (Fitzgerald, 1995).


Figure 1. Eastern Tent Caterpillar developmental stages.
A. Eastern Tent Caterpillar egg mass on cherry branch.
B. Eastern tent caterpillar larvae on tent surface.
C. Eastern Tent Caterpillar larvae and trail webbing deposited as larvae leave defoliated tree in search of alternative food sources.
D. Eastern Tent Caterpillar adult moth.
Over recent decades, control of tent caterpillars has been recommended only for aesthetic purposes as early spring defoliation has little lasting effect on productivity and survival of most trees (Bessin, 1995). The negative impact on the environment associated with controlling ETC populations with insecticides and the cost were considered more significant than the seasonal and episodic nuisance of occasional early spring defoliation. ETC populations harbor a number of diseases that reach epidemic proportions as populations rise and typically cause the population to crash after a few outbreak years. Thus, if their aesthetic impact can be tolerated, ETC outbreaks are effectively transient and self-limiting.
Because anecdotal and survey data support a correlation between ETC, black cherry trees and MRLS and little support was developed for other hypotheses, researchers focused on evaluating the cyanide hypothesis from both entomological and veterinary perspectives. Results from veterinary studies are not considered here as my laboratory has only evaluated the cyanide hypothesis from an entomological perspective. Specifically, we sought to address the following issues.
(1) Do ETC larvae concentrate cyanide in insect tissues? This possibility was addressed by measuring cyanide levels in cherry leaves and in feeding larvae.
(2) Does cyanide pass through ETC larvae so that it might be delivered in quantities sufficient to intoxicate horses via ingestion of forage contaminated with insect excrement (frass)? This possibility was addressed by measuring cyanide and cyanogenic plant precursors in the insect gut and in frass.
(3) Do ETC larvae release cyanide into water? It was suggested that larvae in pools and buckets may have delivered cyanide to horses.
(4) What effect does starvation have on cyanide levels in ETC? The spring of 2001 was unusual in that their preferred host trees were defoliated well before ETC larvae completed their development. This forced larvae into pastures in search of alternative food sources.
(5) Do cyanide levels change over the course of ETC development in such a way as to facilitate delivery of the toxin to horses? As described below, the results of studies conducted over the summer of 2001 suggest that ETC larvae are unlikely to deliver significant quantities of cyanide to horses.
Of necessity, our studies were conducted with ETC larvae shipped to us from areas north of Kentucky and fed on local black cherry foliage collected in June. The possibility that our results would differ if conducted earlier in the year cannot be eliminated, although the similar findings of Fitzgerald et al. (2001) make this unlikely.
Detection of cyanide in Eastern Tent Caterpillars
To evaluate the concentration of cyanide in ETC larvae, insects from Maine and Vermont were fed black cherry leaves from central Kentucky in early June. Two assays were established using potassium cyanide (KCN) and the plant cyanogenic glycoside, prunasin, to standardize the assay. The first assay was based on sodium picrate paper test. The sodium picrate assay has been used for approximately 100 years to detect gaseous cyanide from a number of sources, including homogenates of plant or insect tissues (Dahlman and Johnson, 1980; Brinker and Seigler, 1989). This assay is recognized to be robust but not as sensitive as other methods and not strictly quantifiable. Insects were dissected to investigate the levels of cyanide in the three major regions of the lepidopteran gut, the foregut, midgut and hindgut with the rest of the insect also homogenized and assayed (carcass).
In this initial test, cyanide was readily detected in leaves and in the insect foregut but was almost undetectable in the midgut, hindgut, frass and carcass. Little digestion occurs in insect foreguts (crop), and the ingested plant material in these feeding ETC larvae was visibly green. These results are consistent with those reported by Fitzgerald et al. (2001), who measured cyanide levels of black cherry leaf tissue averaging 1,131 and 3,032 ppm in stems and fresh leaves respectively, an average of 631 ppm in foreguts and 14 ppm in the midgut. Similarly, cyanide levels in frass ranged from 20 to 85 ppm in fresh and dried frass, respectively. By way of comparison, mammalian toxic doses, in terms of LD50, range from 1 to 7 mg/kg body weight (Faust, 1994).
In plants, cyanide is stored as cyanogenic glycosides with enzymes released when the tissue is crushed or ingested that break down the cyanogenic glycosides to release cyanide. The release of cyanide is thought to protect the plants from generalist herbivory (Woodward and Bernays, 1977), but many insects are known to specialize on and prefer cyanogenic plants (Brattsten et al., 1983). Black cherry trees (Prunus serotina) store cyanide as prunasin (β-D-mandelonitrile-glucoside) with a β-glucosidase released to cleave the sugar moiety from prunasin to form the intermediate molecule mandelonitrile (Figure 2). Mandelonitrile spontaneously decays under many physiological conditions to release cyanide and benzaldehyde.
Interestingly, ETC larvae are known to regurgitate the partially digested leaves from the foregut as a defensive reaction when attacked by ants (Peterson et al., 1987). Bioassays of the reactions of ants to cyanide and benzaldehyde suggest that only the latter is a deterrent to ant predation. Because cyanogenic glycosides are precursors for cyanide production, assessing the cyanide that could be produced requires the measuring of cyanide, mandelonitrile and prunasin to determine the total cyanogenic capacity. Conventionally, the plant precursors are converted to cyanide through addition of exogenous β-glucosidase with only the cyanide moiety directly quantified. We adopted and standardized the assay of Esser et al. (1993) with cyanide and β-D-mandelonitrile glucoside for this purpose. Conversion of prunasin to cyanide was near quantitative on a molar basis in standard curves and in test assays with known amounts of cyanide or prunasin in homegenates of insect tissues.
Quantification of preliminary results using the Esser et al. (1993) assay supported our preliminary findings and those of Fitzgerald et al. (2001) in showing that cyanide was found in quantity only in the foregut of feeding larvae. Furthermore, conversion assays to detect prunasin and mandelonitrile demonstrated that the cyanogenic precursors were metabolized in the foregut with negligible quantities detected in the larval midgut or hindgut (Webb and Collins, unpublished). I have estimated the amount of cyanide potentially vectored by Eastern Tent Caterpillars relative to that available through ingestion of plant material. Insect foreguts have about 33% of the cyanogenic capacity of plant tissue on a weight basis. The foregut is about 15% of the total body weight of a feeding larvae, so ETC larvae have about 5% of the cyanide concentration of leaves. ETC frass had about 1% or less of the cyanide levels found in black cherry leaves. In other words, a horse would have to ingest 20 times more larvae and 100 times more frass than black cherry leaves to accumulate an equivalent dose of cyanide. These results indicate that the potential for ETC to vector cyanide to other animals, including horses, is significantly less than that potentially delivered through direct ingestion of plant material.
We have gone on to consider some other factors. We have starved larvae and determined that by 6 hrs of starvation, 9 of 10 larvae had emptied their foreguts of food and cyanide. We assayed feeding larvae for their ability to release cyanide into water and found that submerging larvae of ~1 g in 50 ml of water did not result in the release of cyanide at levels detectable in our assays. Similarly, larvae in the physiological state known as ‘wandering’, in which they search for a pupation site, had no detectable levels of cyanide. These wandering larvae are most noticeable on the ground and most likely to come into contact with horses. Taken together, these studies suggest that only larvae which have been feeding recently on leaves could vector cyanide and further support the conclusion that ETC are unlikely to vector significant quantities of cyanide to horses.

Figure 2.Breakdown of prunasin to release cyanide. The plant cyanogenic glycoside, prunasin, is digested byß-glucosidases to produce mandelonitrile (and glucose). Under neutral and basic conditions, mandelonitrile decays to produce benzaldehyde and the cyanide ion.
There is a possibility that ETC larvae are toxic through some other mechanism that is unrelated to cyanide levels. Again, the data are not available in which ETC larvae have been fed to horses to assess this possibility. However, there is at least one study in which ETC larvae were dried, homogenized and used as a dietary source of protein for rodents. In this study, dried ETC homogenates were fed to rats at concentrations up to 61.5% of dietary nitrogen (Finke et al., 1989). At the two highest doses, diarrhea symptoms were observed and dissection revealed intestinal abnormalities that were suggestive of toxicity. The tent caterpillar meal also seemed less palatable than other insect meals. However, it seems unlikely that horses would have ingested ETC larvae in quantities that were required to induce toxicity in rodents or in anything approaching the levels of dietary nitrogen used in the rodent study.
Other theories related to Eastern Tent Caterpillars As the likelihood that ETC larvae had delivered cyanide to horses decreased, other hypotheses were developed that may explain the relationship between MRLS and ETC. These are briefly described below.
NUTRIENT FLOW OR ‘FRASS-FUNGUS’ HYPOTHESIS
This hypothesis, developed in concert with Dr. Newman of Venture Labs, posits that frass deposited by ETC larvae onto pastures promoted growth of some fungi during early April. The freeze of April 17 and 18 then acted upon the fungi to induce mycotoxin production. When frass-contaminated forage was ingested by horses, the mycotoxins produced by the fungi in the frass could be the proximate cause of MRLS.
DISEASE VECTOR HYPOTHESIS
The disease vector hypothesis posits that a nonconventional infectious agent was transmitted from ETC larvae to horses. There are many insects that transmit disease, most notably those such as mosquitoes and biting flies that are blood-feeders.
Other insects, notably cockroaches and houseflies, transmit diseases through contamination of foods and surfaces. An infectious cause of MRLS was ruled out, in part because of the simultaneous onset of the disease across a wide area. While most infectious diseases do not have this etiology, the appearance of insect-vectored diseases is closely associated with temporal occurrence and exposure to the insect vector. It follows that it might be possible for insects to vector a disease almost simultaneously across a widespread area if the occurrence of the insect is transient. The outbreak of ETC larvae in 2001 provides some of the conditions consistent with the disease vector hypothesis.
IMMUNE SUPPRESSION HYPOTHESIS
Lepidopteran larvae are known to cause allergic reactions among entomologists, which can sometimes be severe. The most common toxicity associated with ETC larvae is a mild irritation of the skin. There is some evidence that the cellular immune system of mares was suppressed (Harrison, personal communication) suggesting that the syndrome involved an insult to the immune system.
This is also consistent with the opportunistic occurrence of bacteria in the placenta of early foals (40-100 days gestation) lost to the syndrome. Thus it seems possible that exposure to ETC larvae may have directly or indirectly affected the immune systems of mares and contributed to MRLS.
ESTROGEN/ANTIESTROGEN HYPOTHESIS
In preliminary analyses of estrogenic and antiestrogenic acitvity, ETC larvae, frass and cherry leaves had significant amounts of anti-estrogenic activity (K. McDowell, personal communication). Based on these data Dr. McDowell has formulated the hypothesis that antiestrogens associated with ETC or cherry leaves may contribute to MRLS. Antiestrogens are known to be capable of inducing abortions.
Although some data support each of the hypotheses described above, it is not possible to evaluate them critically or to focus on one as most likely because all lack critical data. Therefore, my laboratory and colleagues in this work have chosen to focus on the more fundamental question of whether ETC larvae contributed to MRLS. In this way, we make no assumptions as to the mechanism through which ETC may be involved, but simply ask the question of whether they are involved and take measures during the experiment that may lead us to focus on one or more specific hypotheses in subsequent trials and experiments.
Manipulation of direct and indirect exposure to ETC
In the spring of 2002, the association between ETC and MRLS will be investigated with some efforts to mimic some environmental conditions that were suspected to contribute. Rather than design experiments to test each hypothesis, we plan to manipulate the level of exposure of mares in a susceptible stage of pregnancy to ETC larvae and/ or their excrement. We plan two experimental treatments and a control with the experiment designed such that induction of MRLS at levels seen in 2001 would produce a statistically significant result in a single trial. We plan two trials with attempts to mimic the environmental insult thought to be linked to MRLS (the hard freeze) during the second trial.
In the direct exposure to ETC trial, we plan to expose mares between 40 and 100 days gestation to a minimum of 20,000 larvae in small pens for 6 hrs/day over a 10 day period in two separate trials. In the indirect exposure trial, we plan to seed plots with frass from the same number of larvae over the same time period. A control group is included that will receive identical handling but no insect treatments. The hard freeze will be mimicked by chilling frass and/or insects to -2o C overnight prior to delivery to the experimental plots.
It is impossible to replicate all of the environmental conditions, but this experiment has been developed in an attempt to address those two factors, ETC and temperature, that are thought to be involved.
MRLS in a historical context
In 1980 and 1981, phenomena similar to MRLS were reported although records suggest that the severity of the syndrome was less than that experienced in 2001. These years were also notable for their unusual spring weather and high populations of ETC. If a similar causative agent(s) contributed to the equine abortions of 1980 and 1981, then one must assume that the syndrome has been episodic, but unrecognized, for some time, perhaps over 200 years. If so, MRLS did not prevent the establishment and flourishing of the equine industry in central Kentucky and the historical record suggests that future episodes will be infrequent and have a modest impact on the industry over the long term.
Of course, as events of 2001 attest, some years may be very difficult. The prospect that MRLS may be correlated with identified environmental conditions such as ETC population levels and spring weather conditions opens the potential for the equine industry to take preventative measures even in the absence of a known cause. This is the rationale for establishing the MRLS Environmental Monitoring Program at the University of Kentucky. If factors correlated with the occurrence of a disease can be identified, it is possible to control the spread of disease even without knowledge of the etiological agent.
A major complication in study of MRLS is its episodic nature. One can simply not expect to have an occurrence every year and this is likely to make it particularly difficult to investigate. The limited historical record indicates that MRLS is rare, occurring once or twice every 20 years. A human disease that is in some ways similar is the hemorrhagic fever caused by the Ebola virus.
Although the etiological agent causing this disease has been identified (unlike MRLS), its reservoir and sylvatic mode of transmission is not known, principally because of its episodic and unpredictable occurrence. These circumstances suggest that understanding MRLS may require a sustained effort to elucidate its causes and eliminate its impact on the equine industry.
References
Bessin, R. 1995. Eastern Tent Caterpillar. ENTFACT-423 Dept. Entomology Fact Sheet, University of Kentucky.
Brattsten, L.B., J.H. Samueliian, K.Y. Long, S.A. Kincaid amd C.K. Evans. 1983. Cyanide as a feeding stimulant for the southern armyworm, Spodoptera eridania. Ecological Entomology 8:125-132.
Brinker, A.M. and D.S. Seigler. 1989. Methods for detection and quantitative determination of cyanide in plant materials. Phytochemical Bulletin 21:24- 31.
Dahlman, D.L. and V. Johnson. 1980. Heteromeles arbutifolia (Rosaceae: Pomoideade) found toxic to insects. Ent. News 92:141-142.
Esser, S.A.J.A., M. Bosveld, R.M. vanderGrift and A.G.J. Voragen. 1993. Studies on the quantification of specific cyanogens in cassava products and introduction of a new chromogen. J. Sci. Food Agric. 63:287-296.
Faust, R.A. 1994. Toxicity summary for cyanide. h t t p : //risk.lsd.ornl.gov / tox/profiles / cyanide_f_V1.shtml
Finke, M.D., G.R. DeFoliart and N.J. Benevenga. 1989. Use of a four-parameter logistic model to evaluate the quality of the protein from three insect species when fed to rats. Proteins and Amino Acids, pp. 864-871
Fitzgerald, T.D. 1995. The Tent Caterpillars. Cornell University Press., Ithaca, N.Y.
Fitzgerald, T.D., P.M. Jeffers and D. Mantella. 2001. Depletion of host-derived cyanide in the gut of the eastern tent caterpillar, Malacosoma Americanum. J. Chem. Ecol. 28:241-252.
Kane, E. and Kriby E. 2001. Death in the bluegrass. Equus 287:60-68.
Meyers, D.M. and S. Ahmad. 1991. Link between l-3cyanoalanin synthase activity and differential cyanide sensitivity of insects. Biochimica et Biophysica Acta 1075:195-197.
Peterson, S.C., N.D. Johnson and J.L. LeGuyader. 1987. Defensive regurgitation of alellochemicals derived from host cyanogenesis by eastern tent caterpillars. Ecology 68:1268-1272.
Schroeder, L.A. 1978. Consumption of black cherry leaves by phytophagous insects. The American Midland Naturalist 100:294-306.
Seigler D.S. 1991. Cyanide and cyanogenic glycosides. In: Herbivores: their interactions with secondary plant metabolites, 2E Volume 1: the chemical participants. (Rosenthal G.R. and Berenbaum M.R. eds). Academic Press, NY pp 35- 77.
Smeather, D.M., E. Gray and J.H. James. 1973. Hydrocyanic acid potential of black cherry leaves as influenced by aging and drying. Agronomy Journal 65:775-777.
Veterinary Sciences. 2002. Mare Reproductive Loss Syndrome Web Page http://www.uky.edu/ Agriculture/VetScience/gluck1.htm. Vetter, J. 2000. Plant cyanogenic glycosides. Toxicon 38:11-36.
Woodward, S. and E. Bernays. 1977. Changes in release rates of cyanide in relation to palatability of Sorghum to insects. Nature 270:235-235.
Author: BRUCE A. WEBB
Department of Entomology, University of Kentucky, Lexington, KY, USA
Related topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.