Rumen acidosis
Rumen acidosis: modeling ruminant response to yeast culture
Before this dramatic event, there is subclinical acidosis (5.5 < pH <6.2), which is a frequent situation for high yielding animals receiving diets deficient in fibre and rich in highly digestible substrates formulated to meet high energy requirements. Several drawbacks are associated with subclinical acidosis. Low pH in the rumen over a long period of time inhibits intake and cell wall digestion. This last aspect alters the energy value of the diet, particularly of its forage component.
Moreover, the VFA profile in the rumen fluid is altered with a low acetate:propionate ratio and sometimes a significant accumulation of lactic acid.
One of the outcomes of subclinical acidosis is low milk fat content, which can fall below 3%. Several other diseases are associated with subclinical acidosis, as it is a contributing factor in abomasal displacement, liver abcesses and lameness.
The objective of this paper is an overview of the quantitative relationships that link aspects of rumen acidosis and use of yeast supplementation in ruminant diets, particularly diets fed lactating cows.
Methods
To reach this target we have considered the problem from the viewpoint of statistical modeling. Databases were constructed from experiments involving dietary yeast supplements published in scientific papers. Two databases were built from in vitro experimental data measured with either mono- or mixed cultures of rumen microbes. Two in vivo databases were also built. The first contained data on rumen fermentation and digestion measured in vivo on ruminally- and dual- (rumen and duodenal) cannulated animals. The second database contained trials performed on lactating dairy cows with simultaneous data of digestibility.
Interpretation of these databases was based on a statistical meta-analyses (St Pierre, 2001). The publications were separated into experiments that were individually encoded. The basic statistical model applied to the data was:
Yijk = μ + YEASTi + EXPj + Eijk
Where:
- Yijk: observations
- μ: overall mean
- YEASTi: effect of yeast (yeasts vs control)
- EXPj: influence of experiment j
- Eijk: residual error
All the models were used without weighting the observations.
Results
IN VITRO EXPERIMENTS
Monoculture and co-culture
A limited number of publications described experiments where monocultures of rumen bacteria were performed with or without Saccharomyces cerevisiae. An initial goal was to study the impact of yeast supplements in the media on lactate utilisation by lactate fermenters, either Megasphaera elsdenii or Selenomonas ruminantium (Chaucheyras et al., 1995; Rossi et al., 1995; Callaway and Martin, 1997).
There was a clear dose-dependent increase in the utilisation of lactate, suggesting a role for yeast in decreasing rumen acidosis. Unfortunately, as the measured items and the experimental conditions were very different it was difficult to empirically pool the data on lactate metabolism. Simultaneously the acetate:propionate ratio decreased as is shown in Figure 1.
Interesting experiments were also performed with cocultures of M. elsdenii and Streptococcus bovis, a lactate producer (Chaucheyras et al., 1995). In this work, adding yeast boosted the biochemical activity of the organism. Other trials demonstrated that yeast addition to the medium improved cellobiose digestion by fibrolytic bacteria, either Fibrococcus succinogenes or Ruminococcus albus (Callaway and Martin, 1997). Several assumptions were made to explain the mode of action of the yeast supplements, including provision of soluble growth factors (amino acids, organic acids, vitamins), or possibly by improving the anaerobic conditions.
One of the major difficulties in pooling and interpreting databases of mono- and co-cultures is identifying and controlling the specific experimental influences. This is particularly true when the number of available and (or) complete publications is limited, as is presently the case. A useful way to integrate these data is to create simple mechanistic models of in vitro fermentations to connect these experiments and to improve our comprehension of the mode of action of yeast. As an example, Figure 2 is a diagram of a model suited to test the influence of adding yeast on the lactate producers and lactate utilizors. With this model it was possible to achieve fairly good accuracy between simulated and the experimental data of Rossi et al. (1995).
Mixed rumen mixed cultures
A database was built from publications dealing with the impact of yeast on in vitro mixed cultures in rumen fluid. It contained 21 publications, pooling 49 experiments and 121 treatments. In each experiment there was a control compared to one or several treatments where a yeast supplement was added to the culture (n = 89) or to the donor’s rumen fluid (n = 32). There were two in vitro methods, either in batch (n = 69) or in continuous culture devices (n = 52). For each statistical treatment the provided information were the LS means (yeast vs control), the numbers of treatments (n) and of experiments (Nexp), the value of the residual standard deviation (rsd) and its unit, the probability of the test (P) and the percentage of ‘aberrant treatments’ (a) which presented normalised residuals larger than 2.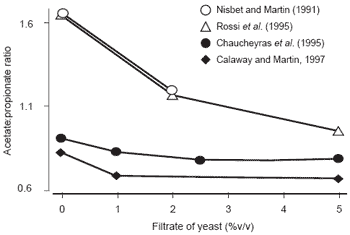
Figure 1. Influence of adding yeast on the acetate:propionate ratio in vitro in co-culture with M. elsdenii.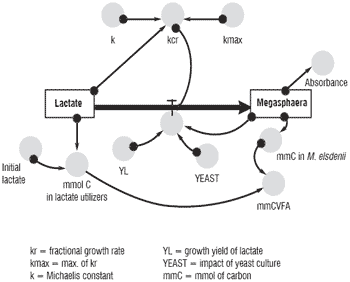
Figure 2. Simple model of the influence of yeast on Megasphaera elsdenii.
There was an increase in pH in response to yeast supplements (6.35 vs 6.32, n = 94, Nexp = 37, rsd = 0.05, P = 0.028, a = 6.4%). Where data were few, this impact was partly confirmed as a trend (n = 82 , P = 0.11) when the analysis was conducted within the experiment and with the volatile fatty acid (VFA) concentration used as a covariate. This trend suggested an eventual favorable effect of yeast on the pH of the medium. To go further in the analysis several sub-bases were built to respond to specific issues.
When only the data with pH<5.5 were considered (Jouany et al., 1998; Lynch and Martin, 2002), the influence of yeast supplementation was more marked (pH increase was 0.055 vs 0.024) but less significant (5.32 vs 5.26, n = 14, Nexp = 6, rsd = 0.05, P = 0.064, a = 7.1%). Pooling the data of Carro et al. (1992), Zelenak et al. (1994) and Lynch et al. (2002) allowed testing where there was any interaction between pH response and the level of cell wall (CW) in the diet or feed. The pH actually increased for feeds or diets having a higher level of CW, however there was no effect of yeast on pH and no interaction between CW and yeast.
There was no effect of treatment on VFA concentrations or production (66.38 vs 65.85, n = 95, Nexp = 36, rsd = 5.0 mM, P = 0.62, a = 5.3%).
The acetate:propionate molar ratio was slightly decreased, but this effect was not significant (2.99 vs 3.05, n = 98, Nexp = 38, rsd = 0.20, P = 0.131, a = 5.1%). The proportions of the isoacids were not altered by yeast supplementation (6.33 vs 6.06, n = 38, Nexp = 15, rsd = 0.36%, P = 0.31, a = 9.5%).
Also, there appeared to be no influence of yeast supplementation on lactic acid concentration in the medium (0.646 vs 0.667, n = 32, Nexp = 11, rsd = 0.105 mM, P = 0.603, a = 3.1%). Similarly, there was no effect when lactic acid concentration was corrected by the VFA concentration, the two items being linked.
The molecular hydrogen status did not seem to be altered by yeast. Effectively CH4 content or production (13.6 vs 13.4, n = 58, Nexp = 23, rsd = 1.3 mM CH4, P = 0.576, a = 1.7%) and H2 content or production (0.444 vs 0.440, n = 44, Nexp = 17, rsd = 0.060 mM H2, P = 0.842, a = 4.6%) were not significantly modified by adding yeast. For CH4 there was a positive correlation with VFA, however there was no effect of yeast when VFA were considered as a covariate. Globally there was no influence of yeast on NH3 content or production (154.4 vs 151.0, n = 55, Nexp = 21, rsd = 10.6 mg N-NH3/L, P = 0.275, a = 7.3%). However, as the experiments measuring NH3 levels lower than 100 mg N-NH3/L were selected, there was a significant increase in response to yeast (72.1 vs 57.8, n = 21, Nexp = 7, rsd = 5.9 mg N-NH3/ L, P = 0.002, a = 0%).
Microbial N production tended to be increased by adding yeast (837.4 vs 791.0, n = 17, Nexp = 8, rsd = 52.1 mg microbial N/d, P = 0.107, a = 11.7%). Also the efficiency of microbial growth tended to be increased by yeast (21.8 vs 20.4, n = 17, Nexp = 8, rsd = 1.48 g microbial N/kg RFOM, P = 0.099, a = 11.8%).The number of protozoa in the medium was unaffected by yeast (4.48 vs 4.53, n = 32, Nexp = 13, rsd = 0.16 log10 protozoa/ml, P = 0.494, a = 6.25%).
In contrast, the total number of viable bacteria was significantly increased by yeast (8.67 vs 8.57, n = 26, Nexp = 9, rsd = 0.08 log10 bacteria/ml, P = 0.009, a = 0%). Moreover, on a larger number of data, the number of cellulolytic bacteria was significantly increased by yeast supplementation (7.65 vs 7.20, n = 32, Nexp = 12, rsd = 0.32 log10 bacteria/ml, P = 0.002, a = 6.2%). All these data suggested an increase of microbial proliferation by adding yeast in vitro. The apparent degradability of substrate dry matter was not significantly increased by yeast addition (58.1 vs 57.7, n = 57, Nexp = 22, rsd = 2.7%, P = 0.613, a = 7.0%). In contrast, on a smaller set of data the degradability of NDF was improved by yeast supplementation (53.3 vs 50.3, n = 32, Nexp = 15, rsd = 4.2%, P = 0.062, a = 6.25%). This last aspect is consistent with the above cited results on the number of bacteria, particularly the cellulolytic species.
IN VIVO EXPERIMENTS
Rumen fermentation and digestion
In order to quantify the effects of yeast on in vivo ruminal fermentation we pooled into a database the results of 55 publications corresponding to 78 experiments and 186 treatments. This database was much larger than the in vitro database. Yeast cultures were from at least eight different commercial preparations. Half of the trials (28 of 56) used Yea- Sacc1026® or a source of strain 1026. In some papers, two yeasts were compared. In all the papers, some rumen parameters were available, at least pH and/or VFAs. Some additional data were noted when available such as in vivo dry matter (DMD) or organic matter (OMD) digestibility, and in situ measurements. There was a lack of basic information on either yeast concentration, or live weight of the animals, or dry matter intake, or chemical analysis of the diet in some of the studies. The yeast effect was tested with the basic model including the trial and yeast effects.
There were non-significant increases in response to yeast supplements in pH (6.341 vs 6.320, n = 168, Nexp = 70, rsd = 0.015, P = 0.288, a = 8.3%), in VFA concentrations (99.1 vs 97.8, n = 156, Nexp = 64, rsd = 75.8 mM, P = 0.386, a = 8.8%), in the acetate/propionate ratio (3.24 vs 3.17, n = 163, Nexp = 69, rsd = 0.078, P = 0.110, a = 3.7%), and a nonsignificant decrease in lactic acid concentration (1.38 vs 1.45, n = 39, Nexp = 15, rsd = 0.417, P = 0.729, a = 10.3%). Except for the pH and the acetate/ propionate ratio, these data were fairly similar to the in vitro responses.
Yeast tended to increase organic matter digestibility (71.4 vs 70.8%, n = 66, Nexp = 31, rsd = 3.23, P = 0.148, a = 9.0%). There was a similar trend for increased in situ DM degradability (55.9 vs 55.0%, n = 75, Nexp = 32, rsd = 7.12, P = 0.145, a = 5.3%), which was interestingly related with rumen pH within (w) the experiment (isDMDw = 10.0 pHw, n = 72, R2 = 26%, rsd = 1.8%, a = 4.0%).
Otherwise it must be stressed that when the 23 treatments having less than 40% dietary concentrate were considered, there was a good fit and a significant increase of OMD (64.6 vs 63.2%, n = 23, Nexp = 11, rsd = 1.33, P = 0.033, a = 0%). There was no statistical influence of yeast on whole tract digestibility of NDF (53.5 vs 54.0%, n = 47, Nexp = 22, rsd = 2.19, P = 0.522, a = 8.5%) and ADF (48.4 vs 47.2%, n = 37, Nexp = 16, rsd = 5.7, P = 0.555, a = 5.4%). This last aspect did not confirm the in vitro data.
When the yeast concentration was available in the paper or could be estimated because the yeast description was sufficient, we used a model including the trial effect and the yeast dose. It was expressed as log10(CFU/100 kg of live weight) because the database concerned cattle and sheep. Globally, similar conclusions could be obtained with this second approach. Nevertheless, it should be stressed that for pH, yeast dose is at the limit of the level of significance: pH = 6.32 + 0.00556 log10 (yeast concentration) (n = 147, Nexp = 64, rsd = 0.032, P = 0.086, a = 8.2%) This increase in pH value in response to yeast supplementation is consistent with the in vitro data.
In order to test whether some strains are more effective than others, we also studied two subdatabases concerning the two most studied strains: Yea-Sacc1026®, and Diamond V. There was no statistical effect of the yeast whatever the sub-database concerned. In the residual of the negative equation linking pH and VFA concentration including a trial effect, the yeast effect was statistically significant for the whole database (P = 0.024) and for the Yea- Sacc1026® database (P = 0.034).
To look for any optimum yeast dosage, a dose effect has been tested on the 10 experiments where three levels were given. There was no dose effect on rumen pH, rumen volatile fatty acids, acetate:propionate ratio or ammonia concentration.
Responses of lactating cows
The data base was constituted from 35 publications pooling 40 experiments and 122 treatments (3272 cows) to quantify the effects of yeast culture on dry matter intake (DMI), raw milk yield (RMY), milk fat content, milk protein content, and body weight change (BWC) of dairy cows. The choice of the data was based on the fact that industrial fermentation processes have rapidly changed since the first publications, consequently only the recent data were considered. The yeast were partitioned among different products (DiamondV-XP: 16 groups; Yeast+: 13 groups, Yea-Sacc1026®: 16 groups, others: 21 groups (Cell-Con, Western 2x225, Levucell), which were pooled because of a low number of groups). Doses used largely differed according to the type of products. Diets were representative of most of the rations used in early- and mid-lactation with high concentrate diets for high producing cows. Most diets were fed as TMRs, once or twice a day. Because literature suggested that effects can differ according to stage of lactation, we coded this as a factor with three levels: EL for experiments starting from calving or during pregnancy and ending before 70 DIM (peak), ELML for experiments starting before peak and ending during mid-lactation, and ML for experiments starting past peak of lactation.
Data recorded for animal response to yeast supplement were dry matter intake (DMI), raw milk yield, milk fat content (MFC), milk protein content, and body weight change (BWC). Simultaneously, data on NDF, ADF and CP digestibility were recorded. The basic model was applied and three others were also used:
Yijk = μ + YEAST + EXP + E ijk
to test the effect of each type of yeast.
Yijk = μ + YEAST + stage of lactation + YEAST * stage of lactation + Eijk
to test the effect of yeast in interaction with stage of lactation (EL, ELML, or ML).
Yijk = μ + YEAST + %Conc + YEAST * Conc + Eijk,
to test the interaction between yeast and %Conc, where %Conc is the percentage of concentrate in the diet.
There was a trend (P = 0.08) toward increased raw milk yield by 1.3 kg when all types of yeast and all stages of lactation were taken together (Table 1). No effects of on milk composition, DMI or BW change were observed. ADF digestibility tended to be increased (P=0.15, +2.8%). This last result was consistent with data observed in vitro.
Table 1. Effect yeast on milk performance of dairy cows in experiments (Nexp) realised during early, early-mid, or mid lactation 1.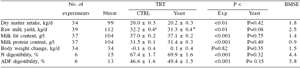
To enlarge the image, click here
1 Data are presented as least square means ± se. RMSE: root mean square error of the model.
When concentrate percentage was included in the analysis, no effect of yeast and no interaction between yeast and percentage of concentrate was significant (data not shown). The role of some other possible interfering factors were also tested. As for concentrate percentage, there was no influence of the dietary NDF or ADF or of the RMY of the control group. In contrast, there was an interesting influence of the milk fat content of the control group (Figure 3). This figure shows that there is an increase in milk fat content in response to yeast supplementation when its value is low, suggesting the presence of subclinical acidosis.
Discussion
Several authors have reviewed the influences of yeast supplements on rumen digestion and animal performance (Ali Haimoud-Lekhal et al., 1999; Jouany, 1999; Lescoat et al., 2000; Garza-Cazares et al., 2001; Chaucheyras-Durand and Fonty, 2002; Robinson, 2002). However, except for the first cited paper, they were largely qualitative or narrative. Otherwise, several authors have noted that there was large variability among publications in the results of yeast influences on ruminant nutrition. That means that by selecting references, it is quite possible to conclude either a positive or a negative effect of adding yeast for any given parameter! Therefore, in order to obtain more relevant and reliable conclusions it was decided to perform a 2-step approach. The first step was to try to be as exhaustive as possible of the published results. The second step was to build databases in each area where a sufficient number of experiments were carried out and published. This is the origin of the four databases mentioned in this paper.
In the current work we had a clear confirmation of the large variability of data among papers. It must be considered that the present approach is only the first step of a more detailed and systematic analysis of all the specific factors which could explain, at least partly, the residual variations of the statistical models. Among these factors there are the strain and the actual level of yeast, comparison between yeast and fungi which were excluded in this work, methodological differences (batch vs continuous culture, adding yeast to the animal vs the fermentor, method of rumen sampling, etc.), dietary specifications, type of animal and level of dry matter intake, type of experimental design, etc. The major technical difficulties that we met in this work were fairly classical, the tables of data were largely incomplete for our purposes and the experimental methodologies and conditions varied largely from one publication to another. Moreover some other specific difficulties were met, the major one being the fact that several of the experiments were available only in form of abstracts and that most of those were very incomplete. This situation can induce bias in the meta-analysis, because non-significant results are generally not presented in an abstract.
For a further step, it would be very important to have more complete information on all these studies. Another problem was that another group of the publications dealing with digestion was very incomplete with, for example, poor information on the specifications of the diet, or substrate used or on the animal characteristics. All these aspects mean that there was obviously a large range in the ‘quality value’ of the various published experiments dealing with yeast response. This suggests that it would be useful to rerun the statistical treatment by weighting the experiments using a ‘quality index’. Another way of weighting the data would be to take into account the residual variations of each experiment. For all these reasons, whatever the conclusions that could be now drawn, it is very important to consider that the all the results presented here cannot be considered as definitive. Lastly, it is probable that non-positive or non-significant results had a lower probability of being published than positive or significant trials, which induced a bias in the conclusions.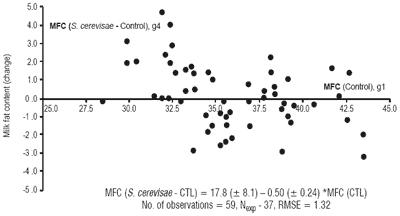
Figure 3. Influence of control milk fat content (MFC) on MFC change with the addition of S. cerevisae in dairy cows.
The method of meta-analysis that we have used is now considered the most suitable (St Pierre, 2001). It is of interest to study the nutritional impact of rumen defaunation (Eugene et al., 2004) and to quantify the biases of rumen mechanistic models (Offner and Sauvant, 2004). This method of statistical modeling has the advantage of splitting variation among and within experiments. The latter is therefore controlled and cannot interfere with the former. However, as such, the meta-design and the model cannot allow us to easily extract and analyze the interactions between the experimental influences and the impact of adding yeast. Obviously this last issue is a big one in the current context. Another big issue in meta-analyses is the way to treat the aberrant treatments and (or) experiments. For the present it was decided to include all the data in the process of interpretation. However it was decided to indicate the proportion of treatments that could be considered aberrant. Moreover, it was also decided to indicate the probability level of the test because in the context of the meta-analysis the threshold value of significance is of importance.
The results were not systematically consistent from one database to another. Some comments have already been noted in the text on this aspect. In the in vitro data it was fairly clear that pH was increased, suggesting a preventative role of yeast toward acidosis. The results were not so clear in vivo, however they were globally consistent. Moreover the interference of either the percentage of concentrate, or the dietary NDF, rapidly degradable carbohydrates, cannot be easily tested due to the scarcity of experiments targeted for that purpose and due to the lack of dietary specification. On this aspect the in vivo database with dairy cows provided an interesting observation, which suggested that yeast supplementation is more efficient in restoring milk fat content when it is low, which is suggests subclinical acidosis.
Further detailed interpretation is also needed regarding VFA and lactic acid production and profiles. On this aspect the results of the in vitro simple culture could not be confirmed with mixed cultures or in vivo. As such, this paper cannot provide useful information about the influence of yeast on hydrogen status and redox aspects. On these points a more detailed interpretation will be necessary.
Microbial concentrations and activities were enhanced by yeast in co-culture and in mixed cultures. These data tended to be confirmed by others, such as the fibre degradability and digestibility data. However, there is a clear lack of in vivo data to confirm these observations on microbial activities. Experiments with duodenally-canulated animals could provide more accurate and extensive knowledge of the impact of yeast on quantitative metabolism in the rumen.
A mechanistic modeling approach was only considered at the level of the co-culture. The first results were encouraging; and it will be useful to develop a mechanistic model of the rumen to take into account the mode of action of yeast on some basic metabolic process such as lactate and VFA metabolism, microbial growth and activities. In this model it will be necessary to include the major principles of thermodynamics (Heijnen and Van Dijken, 1992). This aspect, which has been little explored, could help in understanding any influence of yeast on oxygen/hydrogen balance. As published today, the rumen mechanistic models present some strong limitations (Offner and Sauvant, 2004) and cannot be used to investigate these aspects. Therefore such a project would allow significant progress in methods of modeling the nutritional impact of yeast and other dietary factors in ruminants.
References
Authors: D. SAUVANT, S. GIGER-REVERDIN and P. SCHMIDELYAli-Haimoud-Lekhal, D., P. Lescoat, C. Bayourthe and R. Moncoulon. 1999. Effects of Saccharomyces cerevisiae and Aspergillus oryzae on milk yield and composition in dairy cows: a review. Rencontre des Recherches sur les Ruminants 6:157.
Callaway, E.S. and S.A. Martin. 1997. Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 80:2035-2044.
Carro, M.D., P. Lebzien and K. Rohr. 1992. Influence of yeast culture on the in vitro fermentation (Rusitec) of diets containing variable portions of concentrates. Anim. Feed Sci. Tech. 37:209-220.
Chaucheyras-Durand, F. and G. Fonty. 2002. Yeasts in ruminant nutrition. Experiences with a live yeast product. Kraftfutter 85:146-150.
Chaucheyras, F., G. Fonty, G. Bertin and P. Gouet. 1995. In vitro H2 utilization by a ruminal acetogenic bacterium cultivated alone or in association with an archaea methanogen is stimulated by a probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 61:3466-3467.
Eugène, M., H. Archimède and D. Sauvant. 2004. Quantitative meta-analysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants. Livestock Prod. Sci. 85:81- 97.
Garza-Cazares, F., P. Lebzien and G. Flachowsky. 2001. Effect of Saccharomyces cerevisiae on in sacco dry matter degradability and parameters of rumen fermentation in sheep. Vitamine und Zusatzstoffe in der Ernahrung von Mensch und Tier. 8. Symposium, 26 und 27 September, 2001, Jena/Thuringen, Germany, Friedrich-Schiller- Universitat; Jena; Germany, pp. 501-504.
Giger-Reverdin, S., D. Sauvant, J. Tessier, G. Bertin and P. Morand-Fehr. 2004. Effect of yeast addition in dairy goats diets on rumen metabolism, especially on its buffering capacity. S. Afri. J. Anim. Sci. (submitted).
Grinarii, J.M. and D.E. Bauman, 1999. In: Advances in conjugated linoleic acid research, Vol. 1. (M.P. Yurawecz, M.M. Mossoba, J.K.G. Kramer, M.W. Pariza and J. Nelson, eds). AOCS Press, USA. Heijnen, J.J. and J.P. van Dijken. 1992. In search of a thermodynamic description of biomass yields for the chemotrophic growth of microorganisms. Biotechnol. Bioeng. 39:833-858.
Jouany J.P. 1999. Twenty years of research into yeast culture, now a standard in ruminant diets around the world. Proceeding from Alltech’s Annual European, Middle Eastern and African Lecture Tour, p. 44-68.
Jouany, J.P., F. Mathieu, J. Senaud, J. Bohatier, G. Bertin and M. Mercier. 1998. Effect of Saccharomyces cerevisiae and Aspergillus oryzae on the digestion of nitrogen in the rumen of defaunated and refaunated sheep. Anim. Feed Sci. Tech. 75:1- 13.
Lescoat, P., D. Ali Haimoud-Lekhal and C. Bayourthe. 2000. Effets de Saccharomyces cerevisae et Aspergillus oryzae sur la digestion et le fonctionnement ruminal: étude bibliographique. In: Rème Rencontre des Recherches sur les Ruminants, pp 199.
Lynch, H.A. and S.A. Martin. 2002. Effects of Saccharomyces cerevisiae culture and Saccharomyces cerevisiae live cells on in vitro mixed ruminal microorganism fermentation. J. Dairy Sci. 85:2603-2608.
Nisbet, D.J. and S.A. Martin. 1991. Effect of Saccharomyces cerevisiae culture on lactate utilization by the ruminal bacterium Selenomonas ruminantium. J. Anim. Sci. 69:4628-4633.
Offner, A. and D. Sauvant. 2004. Comparative evaluation of the Molly, CNCPS and LES rumen models. Anim. Feed Sci. Tech. 112:107-130.
Owens, F.N., D.S. Secrist, W.J. Hill and D.R. Gill. 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275-286.
Robinson, P.H. 2002. Yeast products for growing and lactating dairy cattle: impacts on rumen fermentation and performance. In: XII International meeting on milk and meat production in hot climates. Mexicali, Mexico. 2002/10/3-4, (eds).
Rossi, F., P.S. Cocconcelli and F. Masoero. 1995. Effect of a Saccharomyces cerevisiae culture on growth and lactate utilization by the ruminal bacterium Megasphaera elsdenii. Annales de Zootechnie 44:403-409.
St-Pierre, N.R. 2001. Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 84:741-755.
Zelenak, I., D. Jalc, V. Kmet and P. Siroka. 1994. Influence of diet and yeast supplement on in vitro ruminal characteristics. Anim. Feed Sci. Tech. 49:211-221.
INAPG Département des Sciences Animales – UMR INRA–INAPG Physiologie de la Nutrition et Alimentation, Paris, France


United States






