Egyptian probiotic by African Catfish Fingerlings
Evaluation of a new Egyptian probiotic by African Catfish Fingerlings
Published: September 29, 2009
By: A.M. Abdelhamid; Ahmed Ismail Mehrim; Manal Ibrahim El-Barbary; Salah Mohamed Ibrahim3 and Ahmed Ibrahim Abd El-Wahab
Abstract
A preliminary study (120 days) was conducted on African catfish (initial body weight 90 g) to evaluate the beneficial effects of a new patent local probiotic (T-Protphyt 2000) when added to their diet (25% crude protein) at graded levels (0, 1, 2, and 3 g/Kg diet). The diet containing 1 g/Kg (T2) reflected the best growth and feed utilization parameters. Increasing the probiotic level increased fish carcass protein, fat and energy contents, as well as RBCs, WBCs, platelets and A/G ratio but decreased blood proteins. Also, T2 treatment led to improvement of most histometric characteristics of the dorsal muscles of African catfish compared with the control (T1) and other treatments (T3 and T4). The bacterial activity of this probiotic was tested in vitro against nine of pathogenic strains of Gram -negative bacteria (Aeromonas hydrophilla, Pseudomonas aeruginose, Pseudomonas fluorescent, Vibrio.sp, Klebsiella sp, Shigella sp, Salmonella sp, Proteus sp, and Escherichia coli) at two concentrations (120 & 240 mg) compared with oxytetracycline (OTC 30 & 60 mg).The results showed positive effect of the probiotic at the two concentrations against all the tested bacteria.
Key words: Probiotic - Catfish - Growth - Blood - Histometric parameters - Pathogenic bacteria.
INTRODUCTION
Dietary live yeast improved (P < 0.01) fish body weight, reduced muscular fat and serum triglycerides and cholesterol but increased RBC's, Hb, PCV and serum glucose. So, dietary live yeast may improve growth performance and hematological picture in fish (Kobeisy and Hussein, 1995). Moreover, Abdelhamid et al. (2000) reported significant positive effects of the combination of dried live yeast and lacto-sacc on tilapia growth, feed conversion, and nutrients utilization.
The highest level of these separate or combined additives (20 g/Kg) was the best for improving fish body weight and feed conversion. Also, Magouz et al. (2002) and Abou Zied et al. (2003) came to the same conclusion that dietary supplementation of lacto-sacc (0.1% of the diet) produced high growth rate, survival rate and feed and protein utilization of Nile tilapia. Lacto-sacc had also positive effect on economical efficiency of tilapia production. However, Olvera-Novoa et al. (2002) reported that fish fed 25 - 30% (of the dietary protein) yeast diets showed the best growth performance, feed conversion, protein efficiency ratio, nitrogen utilization, incidence cost, and profit index.
Additionally, Khattab et al. (2004) found that Biogen(R) (0.1% of the diet), as a feed supplement, had improved growth performance, feed conversion, chemical composition of the whole fish body as well as the blood profile of Nile tilapia fish. Recently, Allam (2007) found that Pronifer(R) (lactic acid bacteria and its fermentation metabolites) as feed additive at 3% level improved tilapia growth and their blood profile. It reduced fish fat content and alleviated the hazard effects of A. hydrophila on mortality rate. The African catfish Clarias gariepinus is distributed throughout Africa. It is of growing economic value in the African aquaculture industry (Goda et al., 2007; Osman et al., 2007 and Abdelhamid, 2009a).
Probiotics are pure cultures of one or more living microorganisms given in feed that proliferates in the host gastrointestinal (GI) tract. They ensure that the host maintains a beneficial microbial population in the GI tract (Linge, 2005). They confer a healthy effect on the host as significant microbial food supplements in the field of prophylaxis (Geovanny et al., 2007). The research of probiotics for aquatic animals is increasing with the demand for environment-friendly aquaculture. Some probiotics were designed to treat the rearing medium, like biocontrol when the treatment is antagonistic to pathogens or bioremediation when water quality is improved. Most probiotics have been undertaken by isolating and selecting strains from aquatic environment (Gatesoupe, 1999). Also, probiotics have found use in aquaculture as a means of disease control, supplementing or even in some cases replacing the use of antimicrobial compounds (Irianto and Austin, 2002 and Sahu et al., 2008). Since the use of expensive chemotherapeutants for controlling diseases have been widely criticized for their negative impacts (Sahu et al., 2008).Therefore, the objectives of the present study were to evaluate effects of graded levels of a new-local probiotic on African catfish concerning their growth performance, feed utilization, carcass composition, histometric characteristics of the dorsal muscles, blood profile as well as effect of this probiotic on some pathogenic bacteria of fish.
MATERIALS AND METHODS
Experimental management
A field study was conducted to evaluate the effects of dietary inclusion of graded levels of a newly local produced probiotic (T-Protphyt 2000: It is a local product with a patent No. 23593. It consists of 15% zinc salts, 10% inorganic phosphorus, 5% dried fermentation products of Aspergillus oryzae growth, and starch as carrier up to one kilogram. Each gram of this product contains 100 unit of phytase, 75 unit of protease, 25 unit of lipase, and 15 unit of amylase) on African catfish (Clarias gariepins). For this reason, 4 net Hapas (1 x 3 x 1.5 m diameters) were constructed and implanted in 1 Feddan (Egyptian area unit = 4200 m 2) earthen pond in a private fish farm at Tolompat 7, Alriad, Kafr El-Sheikh Governorate, Egypt. The Hapas' net was 1 cm opens, from Tailand. The pond was irrigated via a pump from an agricultural drain. Eighty similar-size catfish were purchased from a private neighbor farm, regardless to their sex, with an average body weight of 90 g.
The feeding trial started on the 20th of July 2008 and ended on the 20th of November 2008 (120 days) using a commercial diet (25% crude protein, from Almorshedy for Trading and Development, Meet Ghamr - Dakhalia - Zagazig Road, Egypt). This commercial diet contained yellow corn, soybean meal (44%), wheat bran, fish meal (65%), corn gluten (60%), lime stone, common salt, dicalcium phosphate, and molasses and had not less than 25% crude protein, 3% crude lipids, 3935 Kcal gross energy/Kg diet, and not more than 5.30% crude fiber, according to the manufacture's formula. Table (1) shows the proximate analysis of the basal diet which was carried out according to AOAC (1995). The diet was ground to add the tested propiotic (at levels of 0, 1, 2 and 3 g/Kg diet, referred to treatments No. T1, T2, T3 and T4, respectively). Molasses at 5% of each diet was used to spread the propiotic, and then all diets were repelleted. The tested diets were offered once daily (10 a.m.) at 5% of the fish biomass at each Hapa. The feed quantity was adjusted periodically according to the actual body weight changes.
Fish performance and quality
Measurements used for the evaluation included fish weight and length and water quality parameters (i.e. temperature using centigrade thermometer, salinity using conductivity TDS meter model 470, pH using pH meter model 340, dissolved oxygen using oxygen meter model 970, all instruments were from Jenway - England).
At the start and at the end of the experiment, fish samples were collected and kept frozen till the proximate analysis of the whole fish body according to AOAC (1995).Their gross energy contents were calculated according to NRC(1993).
At the end of the experiment some fishes from each treatment were sacrificed and fish dorsal muscles were sampled. Samples were fixed in 10% neutralized formalin solution to histometric examination according to Pearse (1968).
Blood Parameters
At the end of the experiment blood samples were collected from the fish caudal peduncle of the different groups. Adequate amounts of whole blood in small plastic vials containing heparin were used for the determination of hemoglobin (Hb) using commercial colorimetric kits (Diamond Diagnostic, Egypt). Also, total erythrocytes (RBCs), platelets and total leucocytes (WBCs) were counted according to Dacie and Lewis (1995) on an Ao Bright - Line Haemocytometer model (Neubauer improved, Precicolor HBG, Germany). Other blood samples were collected and transferred for centrifugation at 3500 rpm for 15 min to obtain blood plasma for determination of plasma total protein according to Gornall et al. (1949), albumin according to Weichsebum (1946) and globulin by difference according to Doumas and Biggs (1972).
Bacterial strains
Nine of Gram-negative bacteria, namely Aeromonas hydrophilla, Pseudomonas aeruginose, Pseudomonas fluorescens and Vibrio.sp in addition to the Enterobacteriaceae(Klebsiella, Shigella, Salmonella, Proteus, and Escherichia coli ) were used in this study, these bacteria were isolated from Nile tilapia fish and identified by specific media and biochemical testes, except Aeromonas hydrophilla that was identified by primed polymerase chain reaction (PCR).
Assessment of antibacterial activity of the probiotic
Powder samples of probiotic and (OTC) antibiotic (because of its wide antibacterial spectrum and high potency, OCT is the most commonly used antibiotic against various diseases caused by Gram-negative and Gram-positive bacteria in fish farming) were suspended in sterile water and used at two concentrations each (120 & 240 mg of probiotic and 30 & 60 mg of OTC). Four wells were punched with a cork borer (6 mm in diameter) in plates of nutrient agar (NA) freshly seeded with 0.1 ml of 24 hr old of each tested bacterial cultures. Different concentrations of probiotic and antibiotic were put into the wells, left one hr to allow diffusion, plates were incubated for 24hr at 37oC. The diameter of clear zones surrounding the wells were measured and recorded expressing the antibacterial activity.
Statistical analysis
The obtained data were statistically analyzed using SAS (1996) procedures for personal computer. When F-test was positive, least significant difference (Duncan, 1955) was calculated for the comparisons among means.
RESULTS
Experimental diet
The chemical analysis of the basal diet used in the present experiment is given in Table 1. Calculated gross energy based on factors of 5.65, 9.45 and 4.11 Kcal/g protein, fat, and carbohydrate, respectively according to NRC (1993) was 395.5 Kcal/100 g, protein/energy (P/E) ratio was 58.76 mg/Kcal.
Table 1: Proximate chemical analysis of the basal diet (% as fed).
Moisture | Crude protein | Ether extract | Total carbohydrates | Ash |
9.01 | 23.24 | 2.87 | 57.67 | 7.21 |
Water quality criteria:
Water parameters were measured twice daily (10 a.m. and 10 p.m.).There were no significant differences among Hapas' water throughout the experimental period because all Hapas were in the same pond. Therefore the means of the whole period are given in the following Table 2.
Table 2: Water quality parameters (means + SE) of the whole experimental period, regardless to the treatments.
Temperature, oC | Salinity, ‰ | pH value | Dissolved oxygen, mg/l |
20.61 + 1.078 | 2.778 + 0.038 | 8.370 + 0.186 | 7.475 + 0.225 |
Growth performance
Growth performance parameters illustrated in Table 3 revealed that T2 (1 g T-Protphyt 2000/Kg diet) was the best among various dietary treatments concerning body weight gain, daily body weight gain, RGR, SGR and condition factor as well as PER, and PPV (even feed conversion ratio which not given in the Table).Yet, all data in this Table are lower than those in literature (El-Haroun, 2007) probably for low dietary protein and fat determined (23.24% and 2.87, respectively) as well as for poor experimental rearing conditions, e.g. stocking rate, feeding rate and frequency and using Hapas which restricted the fish growth and negatively affected feed conversion and nutrients utilization. Therefore, the evaluation will continue in a serial paper under other good ambient conditions.T2 gave significantly (P ≤ 0.05) the highest final body weight and condition factor. However, all supplemental diets with the tested probiotic (T2, T3 and T4) reflected better results than the control (T1). T4 did not differ significantly (P ≥ 0.05) than either T2 or T3.
Table 3: Growth performance of catfish after 120 days of feeding the experimental diets (means + SE).
Items | T1 | T2 | T3 | T4 |
Final body weight, g Body weight gain, g Daily weight gain, mg Relative growth rate*% Specific growth rate**, %/d Final body length, cm Condition factor Survival, % Protein efficiency ratio*** Protein productive value,% **** Energy utilization, %***** | 144 54.0 450 0.351NS+0.046 0.250NS+0.029 26.67b+0.876 0.647c+0.047 100 0.314 5.300 3.443 | 162 72.0 600 1.131NS+0.242 0.619NS+0.095 29.90ab+0.850 0.710a+0.023 100 0.387 7.238 4.282 | 150 60.0 500 1.017NS+0.135 0.582NS+0.054 30.30a+0.569 0.651b+0.006 100 0.342 6.360 3.887 | 148 58.0 483 0.793NS+0.083 0.485NS+0.040 29.33a+0.333 0.653abc+0.024 100 0.328 6.322 4.294 |
a-c: means in the same row with different superscripts are significantly (P ≤ 0.05) different.
NS: not significant at P ≥ 0.05
*RGR = (Final body weight - Initial body weight)/Initial body weight.
**SGR = (In final weight - In initial weight) x 100/experimental period.
***PER = Body weight gain/consumed feed protein.
****PPV = Retained protein x 100/consumed feed protein.
*****EU = Retained energy x 100/consumed feed energy.
Carcass composition
Proximate chemical analysis of the whole fish body at the start and at the end of the 120-day experimental period is summarized in Table 4. These data indicated that moisture content was higher and ether extract as well as energy content were lower at start than at the experimental end; otherwise, no remarkable changes were recorded. Concerning dietary treatments, there were slight increases in crude protein and ether extract percentages but lower ash content due to the dietary inclusion of T-Protphyt 2000.
Table 4: Proximate chemical analysis (% fresh basis) of whole fish body at the start and at the end of the feeding period.
Composition | At start | T1 | T2 | T3 | T4 |
Moisture Crude protein Ether extract Ash Growth energy, Kcal/100 g | 75.21 18.57 0.76 4.72 112.1 | 71.28 17.93 4.10 5.60 140.0 | 71.62 18.36 4.48 4.66 146.0 | 72.02 18.58 4.21 4.63 144.8 | 70.60 18.86 5.13 5.32 155.0 |
Histometric examination of fish dorsal muscles
There were no significant (P ≥ 0.05) differences of all histomestic parameters (smallest diameter (µm), mean diameter (µm), smallest/largest ratio, intensity of muscular bundles/mm2, the percentage of muscular bundles area/mm2 and the percentage of connective tissue/ mm2) of African catfish dorsal muscles among all treatments. However, fish fed diet supplemented with commercial probiotic T-Protphyt 2000 at level of 1g/Kg diet (T2) realized slight improvement of these histometric characteristics of fish dorsal muscles compared with the control (T1) or other treatments (T3 and T4). It is of interest to note that, T2 treatment realized the best growth performance (Table 3) and carcass composition (Table 4) of fish compared with the control and other treatments. This means that supplementation of commercial probiotic T-Protphyt 2000 at level of 0.1% to fish diets led to improvement of most histometric characteristics of the dorsal muscles of African catfish (Table 5 and Figs.1- 4).
Table 5: Effect of dietary supplementation of T-Protphyt 2000 on histometric characteristics of dorsal muscles of catfish (means ± SE)
Treat. | T1 | T2 | T3 | T4 |
Smallest diameter (µm) | 30.40NS ±2.03 | 38.40 NS ±1.59 | 31.20 NS ±2.57 | 34.00 NS ±1.78 |
Largest diameter (µm) | 36.40 NS ±1.72 | 43.60 NS ±2.03 | 40.00 NS ±3.09 | 41.20 NS ±1.85 |
Mean diameter (µm) | 33.40 NS ±1.50 | 41.00 NS ±1.64 | 35.60 NS ±2.69 | 37.60 NS ±1.63 |
Smallest/Largest ratio | 0.84 NS ±0.06 | 0.88 NS ±0.03 | 0.78 NS ±0.04 | 0.83 NS ±0.03 |
Intensity of muscular bundles/mm2 | 586.67 NS ±92.66 | 586.67 NS ±92.66 | 679.33 NS ±92.66 | 679.33 NS ±92.66 |
% of muscular bundles area*/ mm2 | 58.77 NS ±19.07 | 69.63 NS ±4.80 | 57.66NS ±8.67 | 69.63 NS ±4.80 |
% of connective tissue**/ mm2 | 41.23 NS ±19.07 | 30.37 NS ±4.80 | 42.34 NS ±8.67 | 30.37 NS ±4.80 |
NS: not significant at P ≥ 0.05.
* % of muscular bundles area / mm2 = ([3.14 X (mean diameter/2)2] X Intensity of muscular bundles/mm2) X 100, whereas: the muscular bundles were considered in approximately circular shape.
** % of connective tissue / mm2 = (1- muscular bundles area, mm2) X 100
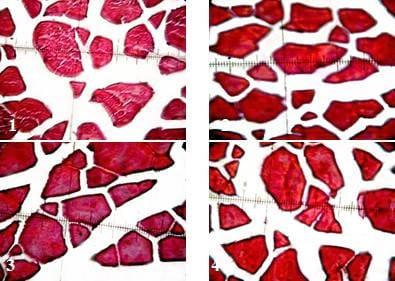
Figs. (1 to 4): Showing cross-section of muscular bundles and interstitial connective tissue of the dorsal muscles of catfish in the 1st, 2nd, 3rd and 4th treatments respectively. (X 400, H & E stains)
Blood profile
Data of hematological and biochemical parameters are given in Table 6, which clears that there were no significant (P ≥ 0.05) effects of the tested probiotic on hemoglobin (Hb) content and white blood cells count (WBCs) comparing with the control. Yet, there were significant (P ≤ 0.05) positive effects on platelets count and A/G ratio and negative effects on total protein and globulin contents; otherwise, no clear trend was recorded for the effect of probiotic inclusion levels. T4 did not differ significantly (P ≥ 0.05) with T3 in most parameters, except A/G ratio, but differ (P ≤ 0.05) with T2 in both red blood cells count (RBCs) and A/G ratio. T3 did not differ significantly (P ≥ 0.05) with T2 in platelets count and A/G ratio. The increased A/G ratio is related to the decrease in globulin values.
Table 6: Hematological and biochemical analysis of catfish blood after 120 days experimental feeding (means + SE).
Items | T1 | T2 | T3 | T4 |
Hemoglobin, g/dl RBCs, x 106/ml WBCs, x 103/ml Platelets, /ml Total protein, g/dl Albumin (A), g/dl Globulin (G), g/dl A/G ratio* | 6.80NS+ 0.40 2.60bc+ 0.10 32.50NS+ 2.50 52.50b+ 7.50 6.15a+ 0.15 1.70b+ 0.10 4.45a+ 0.05 0.382b+ 0.018 | 6.60NS+ 0.10 2.10c+ 0.10 40.00NS+ 5.00 85.00ab+ 10.0 5.60b+ 0.10 1.65c+ 0.05 3.90ab+ 0.20 0.425a+ 0.035 | 6.95NS+ 0.05 2.75a+ 0.15 42.50NS+ 7.50 67.50ab+ 7.50 4.20c+ 0.30 1.45d+ 0.05 2.75b+ 0.25 0.530a+ 0.030 | 7.50NS+ 0.20 2.80ab+ 0.20 47.50NS+ 7.50 100.00a+ 5.00 5.70abc+ 0.20 1.85acd+ 0.05 3.85ab+ 0.15 0.481a+ 0.005 |
a-d: means in the same row with different superscripts are significantly (P ≤ 0.05) different.
RBCs= Red blood cells (Erythrocytes) WBCs= White blood cells (Leucocytes).
*A/G ratio= Albumin /Globulin
Antagonism to pathogens
The results in Table 7 and Fig (5) showed the positive effect of the probiotic at the two concentrations against all the tested bacteria and showed nearly no clear difference between the two concentrations of probiotic. Also, it has a similar effect of the antibiotic (OTC) especially with the pathogenic bacteria, Aeromonas and Pseudomonas, which showed sensitivity towards probiotic, while the Vibrio sp. showed resistance to OTC at the two concentration (Fig. 5,D).
Table 7: The antibacterial activity of aqueous probiotic compared with OTC.
Inhibition zone (mm) of OTC | Inhibition zone (mm) of probiotic | Tested bacterial strains | ||
mg 60 | mg 30 | mg 240 | 120mg | |
36 | 36 | 37 | 32 | A. hydrophilla |
35 | 30 | 38 | 35 | P. aeruginose |
42 | 42 | 44 | 45 | P. fluorescens |
- | - | 36 | 27 | Vibrio. Sp. |
42 | 35 | 30 | 30 | Salmonella. Sp. |
38 | 37 | 35 | 35 | Shigella. Sp. |
30 | 30 | 20 | 21 | Klebsiella. Sp. |
40 | 30 | 22 | 20 | Proteus. Sp. |
38 | 38 | 36 | 34 | E. coli |
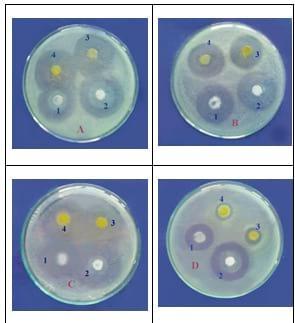
A: A. hydrophilla B: P. aeruginose C: P. fluorescens D: Vibrio. Sp.
1=120mg probiotic 2=240mg probiotic 3= 30mg OTC 4= 60mg OTC
Figure (5): Photographs show the inhibition zones of some bacteria by the action of probiotic compared with OTC.
DISCUSSION
The values of water parameters are within the acceptable ranges recommended for pisciculture (Abdelhamid, 1996 and 2009b and Abdelhakim et al., 2002). However, the optimum growth of African catfish requires 28 - 30oC, < 5 ppt salinity, < 15 mg/l dissolved oxygen, 6.5 - 9.0 pH, and 50 - 100 mg/l hardness in the rearing water (Chapman, 2000).
Craig and Helfric (2002) reported that protein levels in aquaculture feeds generally have an average of 28 - 32% for catfish. Protein requirements are lower for omnivorous fish and for larger fish than carnivorous and smaller ones. Feeding rate affects also fish requirements of protein (Jauncey, 1998).However, Machiels and Henken (1985) reported 40% crude protein, 19.2 KJ DE/g and 13 mg protein/KJ as optimal requirements for C. gariepinus (40 - 120 g). Recently, Eid (2007) recommended a diet containing 25% protein, 6% fat and 72 mg protein/Kcal for adult catfish. Also, El-Gendy (2009) found that the best dietary crude protein and fat levels were 35.9 and 11.7% (465.88 Kcal/100 g), respectively and 77.1 P/GE ratio for the African catfish fingerlings (13 g initial body weight).
Robinson et al. (2009) reported that even though catfish have been cultured for many years, there is still considerable variation in feeding practices on commercial catfish farms. Catfish are generally fed once daily to what is commonly called saturation. Catfish (27 g) feed is generally recommended to contain 28 - 32% protein, starting with a 32% protein feed in spring and change to a 28% feed as the temperature increases.
Abdelhamid et al. (1996) and Attia et al. (2007) confirmed the economical - environmental benefits of using the dietary supplemental microbial - phytase. It increases (P < 0.05) body weight, feed efficiency, carcass %, and serum contents of P, Ca, Mg and Zn. Also, El-Dakar and Gohar (2004) found that growth and survival of Peneaus japonicus post larvae fed probiotic diet was higher than those fed the basal diet. The in vitro study revealed that the probiotic (Bacillus subtilis) decreased the proteolyses activity of the bacterial pathogens (Aeromonas hydrophila, Edwardsiella tarda, and Vibrio proteolyticus). B. subtilis had a positive effect against the pathogens and on reducing the antibiotic susceptibility when presented in culture water or in feed of shrimp. Moreover, Salem et al. (2004) reported that bacterial and yeast probiotics (Bacillus subtilis and Saccharomyces cerevisiae) improve the activity of lactic acid bacteria which could inhibit the pathogenic bacteria (E. coli). Castillo (2008) also concluded that certain Bacillus strains act as probiotic bacteria and block the communication system of Enter pathogens such as Yersinia and Salmonella spp. Yet, Hidalgo et al. (2006) concluded that no significant effects on growth and survival were found following the addition of Bacillus toyaoi, T and B. cereus, E as probiotics to dentex diets.
However, some lactic acid bacteria isolated from the gastrointestinal tract of fish can act as probiotics. These candidates are able to colonize the gut, and act antagonistic against Gram negative fish pathogens. These harmless bacteriocin - producing strains may reduce the need to use antibiotics in future aquaculture (Ringo and Gastesoupe, 1998). Generally, the probiotics actively inhibit the colonization of potential pathogens in the digestive tract by antibiosis or by competition for nutrients and/or space, alteration of microbial metabolism, and/or by the stimulation of host immunity. Probiotics may stimulate appetite and improve nutrition by the production of vitamins, detoxification of compounds in the diet, and by breakdown of indigestible components (Irian and Austin, 2002).
Additionally, Abd El-Rahman and El-Bana (2006) used Micrococcus luteus as a bacterial probiotic which presented in vitro and in vivo antagonistic effects against the pathogenic bacteria Aeromonas hydrophila. The inhibition zone to A. hydrophila was 40 mm in diameter due to M. luteus. The dietary inclusion of this probiotic improved significantly the final weight, weight gain, specific growth rate, feed conversion, protein efficiency ratio erythrocytic counts, hemoglobin content and survival rate of O. niloticas fish. Rollo et al. (2006) reported an improvement in tolerance to acute stress of sea bream fry fed with probiotics. Also, Taoka et al. (2006) indicated that probiotics treatment is promising as an alternative method to antibiotics for disease prevention in aquaculture. Additionally, Attalla (2007) found that supplementation of dietary bacteria or yeast produced significantly better weight gain, specific growth rate, and feed utilization in tilapia cultivation.
Biobuds® and yeast or Biogen® as probiotics led to improving the growth performance, feed conversion, protein efficiency ratio and feed costs for tilapia fingerlings (Mohamed, 2007 and Mohamed et al., 2007, respectively). Moreover, El-Ashram et al.(2008) concluded that, super Biobuds® can improve body gain, survival and enhance resistance to challenge infection. Yet, Abdelhamid and Elkatan (2006) found that dietary supplementation of Biobuds® slightly improved body weight gain but reduced the survival rate of tilapia fingerlings. El-Haroun et al.(2006) and El-Haroun (2007) reported that Biogen® dietary supplementation improved growth performance and feed utilization, carcass protein and fat percentages as well as economical profit in Nile tilapia and catfish culture, respectively. Wongsa and Werukhamkul (2008) came to the same conclusion, since they found that catfish fed diets containing probiotics plus phytase enzyme showed 35% higher weight gain and better feed conversion by more than 25% in comparison to catfish fed control diets in a three-month trial.
However, during the past two decades, the use of probiotics as an alternative to the use of antibiotics has shown to be promising in aquaculture, particularly in fish and shellfish larviculture (Tinh et al., 2008). Recently, Aly et al. (2008a) found that some Bacillus and Citrobacter strains isolated from Nile tilapia (B. pumilus, B. firmus, and C. freundii) showed inhibitory effects against A. hydrophila. Also, Aly et al. (2008b) reported that the probiotic activity of two bacteria (Bacillus subtilis and Lactobacillus acidophilus) was evaluated by its effect on the immune response of Nile tilapia (Oreochromis niloticus), beside its protective effect against challenge infection. Furthermore, their in-vitro inhibitory activity was evaluated. The in-vitro antimicrobial assay showed that Bacillus subtilis and Lactobacillus acidophilus inhibited the growth of A. hydrophila. The B. subtilis inhibited the development of P. fluorescens while L. acidophilus inhibited the growth of Strept. iniae. The B. subtilis and L. acidophilus proved harmless when injected in the O. niloticus. The feed, containing a mixture of B. subtilis and L. acidophilus or B. subtilis alone, showed significantly greater numbers of viable cells than feed containing L. acidophilus only after 1, 2, 3 and 4 weeks of storage at 4oC and 25oC. The survival rate and the body-weight gain were significantly increased in the fish given B. subtilis and L. acidophilus for one and two months after application. The hematocrit values showed a significant increase in the group that received the mixture of B. subtilis and L. acidophilus compared with the control group. The nitroblue tetrazolium (NBT) assay, neutrophil adherence and lysozyme activity, showed a significant increase in all the probiotic-treated groups after 1 and 2 months of feeding, when compared with the untreated control group. The serum bactericidal activity was high in the group that was given a mixture of the two bacteria.
Also, Marzouk et al.(2008) reported that probiotics (B. subtillis and Saccharomyces cerevisae) revealed significant improvement in growth parameters and showed failure in re-isolation of some pathogens. Varley (2008) cited also that probiotics show real benefits in the synergistic effects with the beneficial bacteria in making inroads into improving gut health. So, probiotics may improve the growth performance and immune response of fish (Wang et al., 2008).
The increased count of WBCs may be caused by protein resorption (Merck, 1976). Hypoproteinemia may be due to protein loss, decreased albumin, or to increased globulin (Merck, 1974). An insufficient amount of protein in the diet may lead to a low total protein of blood. This is the case in starvation. In other cases, although adequate protein is being taken in the feed, absorption from the alimentary tract may be defective. This may occur in vitamin deficiencies particularly of the B-vitamins (Varley, 1978).
In the field of physical structure of tilapia muscles, Abdelhamid et al. (2004) found that probiotics (Betafin® and Biopolym®) not only increased body weight, growth rates and total productivity, but also improved muscular protein %, radius of the muscular bundles, total surface area occupied by the muscular bundles/mm2 (least thickness of connective tissues between muscular bundles and thickness of skin and subcutaneous layer), and net return.
CONCLUSION
In conclusion, this preliminary study revealed that this probiotic under study is useful for enhancing fish growth, feed and nutrients utilization, fish chemical composition and muscular structure, besides fish resistance for pathogenic bacteria, i.e. it may be useful also from the economic point of view. Yet, it is to recommend also more research work on this probiotic under various conditions, e.g. different fish species, initial weight, stocking rates, feeding rates, dietary protein levels and sources, ... etc.
REFERENCES
Abdelhakim, N.F., M.N. Baker and M.A. Soltan, 2002. Aquatic Environment for Fish culture. Cairo, (ISBN: 977 - 298 - 228 - 5).
Abdelhamid, A.M., (1996). Field and Laboratorial Analysis in Animal Production. Dar Anashr for Universities, Cairo, (ISBN: 997 - 5526 - 47 - 7).
Abdelhamid, A.M., 2009a. Recent Trends in Fish Culture. New Universal Office, Alexandria, (ISBN 997 - 438 - 053 - 3).
Abdelhamid, A.M., 2009b. Fundamentals of Fish Production and Culture. New Universal Office, Alexandria, (ISBN 977 - 438 - 052 - 5).
Abdelhamid, A.M. and M.S.A. Elkatan, 2006. A study on Nile tilapia fingerlings during wintering using dietary addition of Bio-Buds®-2X. J. Agric. Sci. Mansoura Univ., Egypt, 31: 5705 - 5711. (ISSN 1110 - 0346).
Abdelhamid, A.M., A.E. Abd El-Khalek, M.A.A. Mostafa, S.A.A. Gomaah and F.F.M. Khalil, 2004. Effect of using Betafin® and/or Biobolym® as natural additives in producing Nile tilapia fish in poly-culture semi-intensive system in earthen ponds. J. Agric. Sci. Mansoura Univ., Egypt, 29: 3149 - 3162.(ISSN: 1110-0346).
Abdelhamid, A.M.; F.I. El-Hawary; M.M. El-Sawah and W.I. Saber, 1996. Benefits of dietary supplemental microbial-phytase in the broilers production. Proc. Conf. Food Borne Contamination & Egyptian's Health, Mansoura Univ., Egypt, Nov. 26 - 27, pp: 345 - 353. (ISSN: 1110-0346).
Abdelhamid, A.M.; F.M. Khalil and M.A. Seden, 2000. Possibility of using dried live yeast and lacto-sacc in Nile tilapia fingerlings' diets. J. Agric. Sci. Mansoura Univ., Egypt, 25: 4905 - 4911. (ISSN: 1110-0346).
Abd El-Rahman, A.M.M. and L.F.A. El-Bana, 2006. Using of Micrococcus luteus as a probiotic bacteria among cultured Oreochromis niloticus SCVMJ, X(1): 73 - 82. (ISSN: 0004-6361)
Abou Zied, R.M.; A.M. Abd El-Maksoud and A.A. Ali 2003. Effect of virginiomycin or lacto-sacc on the growth performance of Nile tilapia fish during the nursing period. Egyptian J. Nutrition and Feeds, 6 (Special Issue): 481 - 489.(ISSN: 1110-6360).
Allam, H.Y.H., 2007. Physiological effects of some additives on growth, blood constituents and immunity in Nile tilapia (Oreochromis niloticus). Ph.D. Thesis, Fac. of Agric., Assiut Univ., Egypt.
Aly, S.M., A.M. Abd-El-Rahman, G. John and M.F. Mohamed, 2008a.Characterization of some bacteria isolated from Oreochromis niloticus and their potential use as probiotics. Aquaculture, 277: 1 - 6. (DOI:10.1016/j.aquaculture.2008.02.021).
Aly, S.M., Y.A. Ahmed, A.A. Ghareeb and M.F. Mohamed, 2008b. Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish and Shellfish Immunology, 25: 128 - 136.(DOI:10.1016/j.fsi.2008.03.013).
AOAC, 1995. Association of Official Analytical Chemists, Official Methods of Analysis, 16th Ed., Arlington, VA. 1832. (http://www.covance.com/analytical/svc_nutrients_min.php).
Attalla, R.F., 2007. The use of some microorganisms species as growth promoters for Sarotheroden galilacus. Pakistan J. of Oceanography, 3 (1): 25 - 36. (ISSN: 1817-1761).
Attia, F.A.M., H.S. Abd Elhalim and K.A. Ibrahim, 2007. Improving phytate phosphorus utilization by adding phytase to low protein, phosphorus and calcium corn-soybean broiler diets. Agricultural Research J., Suez Canal University, Egypt, 7(3): 1 - 10 (ISSN 1687 - 1049).
Castillo, M., 2008. Blocking pathogens with Bacillus strains. Feed-Mix, 16 (5): 19. (ISSN: 1754-5692).
Chapman, F.A., 2000. Farm-raised channel catfish. Department of Fisheries and Aquatic Sciences, Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, 32611.
Craig, S. and L.A. Helfric, 2002. Understanding Fish Nutrition, Feeds, and Feeding. Department of Fisheries and Wildlife Sciences, College of Vet. Med., Pub. No. 420 - 256.
Dacie, J.V. and S.M. Lewis, 1995. Practical Haematology. 8th ed. Edinburgh, Scotland: Churchill Livingstone.
Doumas, B.T. and H.G. Biggs, 1972. Determination of serum albumin. In: Standard Method of Clinical Chemistry.Vol.7 Edited by G.R. Cooper, New York Academic press.
Duncan, D.B., 1955. Multiple range and multiple F-test. Biometrics, 11:1-42.
Eid, A.M.S., 2007. Nutritional Requirements for Fish Academy of Scientific Research and Technology, Cairo, Egypt.
El-Ashram, A.M.M., M.F. Mohammed and S.M. Aly, 2008. Effect of Biobuds as a commercial probiotic product in cultured tilapia. 8th International Symposium on Tilapia in Aquaculture, Oct., Cairo, Egypt, pp: 1089 - 1096. (ISBN: 978-1-888807-18-9).
El-Dakar, A.Y. and Y.M. Gohar, 2004. Use of Bacillus subtilis in microparticular diets for producing biocecure Peneaus japonicus postlarvae. J. Agric. Sci. Mansoura Univ., Egypt, 29: 6853 - 6871. (ISSN: 1110-0346).
El-Gendy, E.H.I., 2009. Effect of protein and oil levels on growth performance of catfish (Clarias gariepinus). M.Sc. Thesis, Faculty of Agriculture, Suez Canal University, Ismailia, Egypt. (ISSN 1687 - 1049).
El-Haroun, E.R., 2007. Improved growth rate and feed utilization in farmed African catfish Clarias gariepinus (Burchell 1822) through a growth promoter Biogen® supplementation. American Fisheries Society, 137thAnnual meeting, San Francisco, USA, Journal of Fisheries and Aquatic Science, 2 (5): 319 - 327. (DOI: 10.3923/jfas.2007.319.327).
El-Haroun, E.R., A.M.A.S. Goda and M.A. R. Chowdhury, 2006. Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquaculture Research, 37: 1473 - 1480. (DOI:10.1111/j.1365-2109.2006.01584.x).
Gatesoupe, F.J., 1999. Review, the use of probiotics in aquaculture. Aquaculture, 180: 147 - 165. (www.elsevier.nlrlocateraqua-online).
Geovanny, G.R., B.J. Luis, and M.A. Shen, 2007. Probiotics as control agents in aquaculture. Journal of Ocean University of China, 6 (1): 76 - 79. (ISSN:1672-5182). (http://www.ouc.edu.cn/xbywb/).
Goda, A.M., E.R. El-Haroun and M.A. Kabir-Chowdhury, 2007. Effect of totally or partially replacing fish meal by alternative protein sources on growth of African catfish Clarias gariepinus (Burchell, 1822) reared in concrete tanks. Aquac. Res., 38: 279 - 287. (DOI: 10.1111/j.1365-2109.2007.01663.x).
Gornall, A.G., G.J. Bardawill and M.M. Parid, 1949. Method of determination protein in serum blood .J. Biol. Chem., 177: 751.
Hidalgo, M.C., A. Skalli, E. Abdellan, M. Arizcus and G. Cardenete, 2006. Dietary intake of probiotics and maslinic acid in juvenile dentex (Dentex dentex L.): effects on growth performance, survival and liver proteolytic activities. Aquaculture Nutrition, 12: 256 - 266. (ISSN:1353-5773).
Irianto, A. and B. Austin, 2002. Review, Probiotics in aquaculture. Journal of Fish Diseases, 25: 633 - 642. (ISSN: 0140-7775).
Jauncey, K., 1998. Tilapia Feeds and Feeding. Pisces Press Ltd., Stirling, Scotland, 240 p.
Khattab, Y.A.; A.M. Shalaby, S.M. Sharaf, H.I. El-Marakby and E.H. Rizk Alla, 2004.The physiological changes and growth performance of the Nile tilapia Oreochromis niloticus after feeding with Biogen® as growth promoter. Egypt. J. Aquat. Biol. & Fish., 8 (2): 145 - 158. (ISSN: 1110-6131).
Kobeisy, M.A. and S.Y. Hussein, 1995. Influence of dietary live yeast on growth performance and some blood constituents in Oreochromis niloticus. Proc. 5th Sci. Conf. Animal Nutrition, Ismailia, Egypt, 12 - 13 Dec., pp: 417 - 425. (ISSN 1687 - 1049).
Linge, P., 2005. The use of probiotics and yeast derivatives in India. World Poultry, 21(10): 12 - 15. (http://www.worldpoultry.net/article-database/the-use-of-probiotics-and-yeast-derivatives-in india-id1233.html).
Machiels, M.A.M. and A.M. Henken, 1985. Growth rate, feed utilization and energy metabolism of the African catfish, Clarias gariepinus (Burchell, 1822), as affected by dietary protein and energy content. Aquaculture, 44 (4): 271 - 284. (ISSN:0044-8486).
Magouz, F.I.; M.K. Mohsen and A.H. Gooda, 2002. Effect of including some biological feed additives in the diet on growth performance and feed efficiency of Nile tilapia (Oreochromis niloticus). Proc. 2nd Conf. Foodborne Contamination and Egyptian's Health, Mansoura Univ., Egypt, 23 - 24 April, pp: 329 - 339. (ISSN: 1110-0346).
Marzouk, M.S., M.M. Moustafa and N.M. Mohamed, 2008. The influence of some probiotics on the growth performance and intestinal microbial flora of O. niloticus. 8th International Symposium of Tilapia in Aquaculture, Oct., Cairo, Egypt, pp: 1059 - 1071. (ISBN: 978-1-888807-18-9).
Merck, E., 1974. Klinisches Labor. 12. Auflage Merck, Darmstadt.
Merck, E., 1976. Labordiagnostik in der Tiermedizin. Diagnostica Merck. Darmstadt.
Mohamed, K.A., 2007. Effect of using probiotic and yeast as growth promoters in commercial diet of tilapia (Oreochromis niloticus) fingerlings. Agricultural Research J., Suez Canal University, Egypt, 7(3): 41 - 47 (ISSN 1687 - 1049).
Mohamed, K.A.; B. Abdel Fattah and A.M.S. Eid, 2007. Evaluation of using some feed additives on growth performance and feed utilization of monosex Nile tilapia (Oreochromis niloticus) fingerlings. Agricultural Research J., Suez Canal University, Egypt, 7(3): 49 - 54 (ISSN 1687 - 1049).
NRC, 1993. Nutrient Requirements of Fish. National Academy Press, Washington D.C., USA. 114 p.
Olvera-Novoa, M.A., C.A. Martinez - Palacios and L. Olivera - Castillo, 2002. Utilization of torula yeast (Candida utilis) as a protein source in diets for tilapia (Oreochromis mossambicus Peters) fry. Aquaculture Nutrition, 8: 257 - 264. (ISSN:1353-5773).
Osman, A.G.M.; S. Wuertz, I.A.A. Mekkawy, H. Exner and F. Kirschbaum, 2007. Embryo-toxic effects of lead nitrate of the African catfish Clarias gariepinus (Burchell, 1822). Journal of Ichthyology, 23: 48 - 58. (ISSN: 1531-8486).
Pearse, G.W., 1968. Histological effects and diagnostic problems of mycotoxins in poultry. Proc. 25th West States Poult. Dis. Conf., pp.76-79.
Ringo, E and F.J. Gatesoupe, 1998. Review, Lactic acid bacteria in fish: a-review. Aquaculture, 160: 177 - 203. (ISSN: 0044-8486).
Robinson, E.H.; M.H. Li and M.W. Brunson, 2009. Feeding catfish in commercial ponds. Oklahoma State University Cooperative Extension Fact Sheets (SRAC-181), Division of Agricultural Sciences and Natural Resources.
Rollo, A., R. Sulpizio, M. Nardi, S. Silvi, C. Orpianesi, M. Caggiano, A. Cresci, and O. Carnevali, 2006. Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiology and Biochemistry, 32: 167 - 177. (DOI: 10.1007/s10695-006-0009-2).
Sahu, M.K., N.S. Swarnakumar, K. Sivakumar, T. Thangaradjou and L. Kannan, 2008. Probiotics in aquaculture: importance and future perspectives. Indian Journal of Microbiology, 48: 299-308. (DOI 10.1007/s12088-008-0024-3).
Salem, A.Z., M.M.I. El-Adawy, M.S.M. Salem and A.A. Hasan, 2004. Effect of probiotics feed as additives on the activity of isolated and characterized lactic acid intestinal bacteria against Escherichia coli - 10 in sheep. Egypt. J. Nutrition and Feeds, 7 (2): 167 - 184. (ISSN: 1110-6360).
SAS, 1996. SAS/STAT Guide for personal computer. SAS Inst. Cary, N. C. (www.informatik.uni-trier.de/~ley/db/conf/sas/sas98.html - 15k), (ISBN: 3-540-65014-8).
Taoka, Y., H. Meeda, T.Y. Jo, S.M. Kim, S.I. Park, T. Yoshikhawa and T. Sakata, 2006. Use of live and dead probiotic cells in tilapia Oreochromis niloticus. Fisheries Science, 72: 755 - 766. (ISSN: 0919-9268).
Tinh, N.T.N., K. Direckens, P. Sorgeloos, and P. Bossier, 2008. A review of the functionality of probiotics in the larviculture food chain. Marine Biotechnology, 10: 1 - 12. (DOI: 10.1007/s10126-007-9054-9).
Varley, H., 1978. Practical Clinical Biochemistry. 4th Ed., Arnold-Heinemann Publishers (India) Private Limited, New Delhi - 28.
Varley, M., 2008. Managing gut health without antibiotics. Pig Progress, 24(7): 27 - 28. (http://www.pigprogress.net/article-database/managing-gut-health-without-antibiotics id882.html).
Wang, Y.B., Z.Q. Tian, J.T. Yao and W.F. Li, 2008. Effect of probiotics, Enteroccus faccium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture, 277: 203 - 207. (DOI:10.1016/j.aquaculture.2008.03.007).
Weichsebum, T.E., 1946. Method for determination of albumin in serum blood. Amer. J. Clin. Pathol., 16-40.
Wongsa, P. and P. Werukhamkul, 2008. Improving growth performance and FCR in pangasius catfish with a combination of probiotics and phytase enzyme. AQUA Culture Asia pacific Magazine, Sept./Oct., pp: 22 - 23. (http://www.enaca.org/modules/wfdownloads/viewcat.php?op=&cid=13).
Related topics:
Authors:
Mansoura University, Egypt
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.



.jpg&w=3840&q=75)