Changing technologies, ingredients and formulations to replace fish meal in salmonid diets
Although the search for alternatives to fish meal in aquafeeds has been active for the last 30 years (Cho et al., 1974; Dabrowska and Wojno, 1977; Higgs et al., 1978), efforts in the last decade have been intensified on a worldwide scale (Kaushik et al., 1995; Adelizi et al., 1998; Barrows and Hardy, 2001; Lee et al., 2002; Yamamoto et al., 2002).
Multiple factors are driving these efforts including regulatory concerns on the environmental effects of nutrient release (Barrows and Hardy, 2001), the perceived sustainability of feeding fish meal to fish, and the rapidly rising cost of fish meal (McLean and Craig, 2006).
The search for alternatives to fish meal has led in many directions, but plant-derived ingredients and single cell proteins such as yeasts, bacteria, and micro-algae seem to offer the most promising possibilities (Mukhopadhyay and Ray, 1999; Skrede et al., 2002; Ng et al., 2002; Bairagi et al., 2004; Reftsie et al., 2005).
Animal by-products often have phosphorus levels that severely limit dietary inclusion levels due to environmental regulations. In addition, recent Food and Drug Administration regulations in the US concerning the use of mammalian products further discourages the use of mammalian by-products in aquafeeds.
Limitations in inclusion levels of traditional plant products in salmonid feeds have been clearly demonstrated (Ketola and Harland, 1993; Adelizi et al., 1998; Stone et al., 2005). Each of the traditional ingredients, such as soybean meal, corn gluten meal and wheat flour, have specific characteristics that limit inclusion. These include both nonnutritive (e.g., fiber, pigments) and anti-nutritive (e.g., exotic carbohydrates, phytate, gossypol, agglutinins) components (Gatlin et al., 2006).
For these reasons and others, there is still skepticism being expressed concerning the feasibility of feeding plantbased ingredients to carnivorous fish. Contrary to those opinions, several studies have demonstrated that trout fed plant-based diets, without fish meal, can have growth comparable to trout fed fish meal-based diets (Kaushik et al., 1995; Gaylord et al., 2006; Barrows et al., 2007). Total replacement of fish meal with traditional ingredients alone will not support growth equivalent to trout fed fish meal-based diets, however, unless adjustments in formulations are made using enhanced and novel ingredients.
Grains and oilseeds are low phosphorus, sustainable, readily available nutrient sources and will probably be major ingredients in trout feeds of the future. Commercial trout feeds today contain much higher levels of plant ingredients and lower levels of fish meal than just 3-4 years ago, but continued progress in reducing dietary fish meal is limited by the characteristics of available ingredients. Further processing of plant-based ingredients before feed manufacturing, called pre-process modification, can increase inclusion limits.
Plant-based ingredients in future salmonid diets will have improved nutrient profiles, relative to traditional ingredients, with reduced fiber and anti-nutrient contents. Future ingredients will also be improved by starting the modification process with superior cultivars of grains and oilseeds.
Modern genetic tools are being used to produce cultivars of grains and oilseeds with improved nutrient profiles. Grains and oilseeds contain very low levels of phosphorus relative to fish meal, but a high percentage of the phosphorus is unavailable to fish since it is bound to phytate. Cultivars of corn, barley and soybean are available that have phytate levels reduced up to 90%, and support good growth of trout (Sugiura et al., 1999; Wilcox et al., 2000; Raboy et al., 2001; Overturf et al., 2003).
Feeding low-phytic acid (LPA) varieties to trout and other monogastric species will result in additional reductions in the release of nutrients from farms (Sugiura et al., 1999). There are many new cultivars with improved nutrient profiles, both nutritive and non-nutritive, being developed in laboratories across the country.
Development of plant-based ingredients specifically for aquaculture will benefit grain and fish farmers, but it is also likely that many ingredients used in aquafeeds will be co-products from other industries. The push to develop alternatives to fossil fuels has focused on sustainable production of ethanol and bio-diesel. It is well known that these industries will send ripples, or even waves, through the animal feeding industry as the quantity of material that is changed from feed ingredients to feedstock for energy production is increased. This trend seems to be an inevitable challenge for the animal feeding industry, but one for which the salmonid industry has an advantage over other segments of animal production. This challenge can be met through innovative development of alternate protein sources.
Pre-process modification
Ingredients that can replace fish meal in trout feeds need to contain high levels of protein, and thus low levels of starch and other carbohydrates. Grains contain relatively high levels of starch, fiber and other non-starch polysaccharides (NSP), and low levels of protein and energy, compared to fish meal. NSP have no nutritional value to salmonids, since the enzymes required for digestion are lacking, and bacterial fermentation in the intestinal tract is insignificant (Stickney and Shumway, 1974; Lindsay and Harris, 1980).
Feeding diets with increased levels of NSP only results in increased fecal waste, which is an environmental and regulatory problem. Even though starch is poorly utilized by trout and salmon, 10-15% of the diet as starch is needed to bind pellets during extrusion (Hardy and Barrows, 2000). Methods are needed to concentrate nutrients, primarily protein, and reduce anti-nutrient concentrations.
There are three primary ways to enhance the nutrient profile of plant products for fish diets: chemical, mechanical, and biological.
CHEMICAL MODIFICATION
Chemical modification is more often known as ‘wet processing’ and involves solubilization and extraction of specific components. Wheat gluten meal and soy protein concentrates are good examples of products produced by wet processing methods. These products have been prohibitively expensive in the past for use in fish diets except for use in starter feeds. The costs of the ingredients, relative to fish meal, have changed, making them increasingly attractive alternatives to fish meal in diets for multiple life stages.
MECHANICAL MODIFICATION
Mechanical modification involves the physical separation of components by grinding, de-hulling, pearling and other methods that are used in conjunction with sifting or classification of different sizes or densities of particles. The concept behind this approach is that nutrients will be portioned into particles of different sizes, densities or locations in the seeds. There seem to be differences among species of plants in this regard. Rice protein concentrate produced by air classification is commercially available in either 70 or 80% protein products. After grinding, particles are separated by air classification based upon particle density, with protein-containing particles being denser than the carbohydrate particles. The structure of a rice grain seems to be ideal for this process allowing for concentration from ~15% protein to as high as 80%.
Modification of grains and oilseeds using these methods does affect nutrient digestibility. Wheat gluten meal (WG) contains 79% protein compared to only 13% protein in unprocessed wheat. Some soy protein concentrates (SPC) contain 82% protein, compared to 48% protein in solvent-extracted soybean meal (SBM). The apparent digestibility coefficients (ADC) for dry matter (Figure 1) and the ADC for protein (Figure 2) for both wheat and soy increased when concentrates were produced. These ADCs were equivalent to values for a high quality anchovy meal. Barley protein concentrate (BPC) and rice protein concentrate (RPC), are products produced by airclassification.
A similar increase in protein concentration during this mechanical processing was achieved, relative to the increase in protein by wet processing of soybeans and wheat. The ADC for dry matter for BPC increased substantially, from 54.8% to 73.1%, but was still not equivalent to anchovy meal (87%). The dry matter ADC for RPC was also greatly improved compared to the un-concentrated product, from 57.5% to 82.9%, and was equivalent to anchovy meal. The protein ADCs for rice and barley were both improved with concentration by air-classification, but the products are not comparable in protein ADC to anchovy meal (Figure 2). Both the wet and dry methods improved nutrient digestibility, but the wet method resulted in products with higher ADCs than the dry methods. Concentration of plant proteins removes anti-nutrients and non-nutritive components and results in products more like fish meal, thus increasing replacement value for fish meal.
BIOLOGICAL MODIFICATION
The overall goal of biological modification is the same as with mechanical and chemical modification. Removal of the exotic or fermentable carbohydrates and phytate can easily be accomplished with this approach (Ng et al., 2002; Skrede et al., 2002; Reftsie et al., 2005).
Microorganisms consume and convert carbohydrates that are of low value to salmonids and other carnivorous fish into cell mass that will be a digestible source of both protein and energy. Yeast, bacterial and fungal fermentations have been investigated and current results demonstrate great potential for removing anti-nutrients and adding essential nutrients such as protein and amino acids (Mukhopadhyay and Ray, 1999; Bairagi et al., 2004). The type of microorganisms and plant substrates used in the fermentation will determine the nutrient and anti-nutrient profile of the final product. 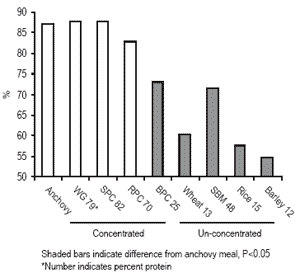
Figure 1. Apparent digestibility coefficients of dry matter for rainbow trout in concentrated and un-concentrated plant ingredients.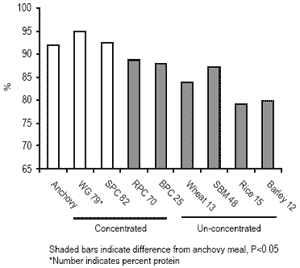
Figure 2. Apparent digestibility coefficients for protein for concentrated and un-concentrated plant ingredients for trout.
Products developed from single cell proteins often contain protein levels of over 50%, and thus are suitable candidates to replace dietary fish meal. Yeast and yeast products can also be a significant source of B-complex vitamins, nucleotides and pigments. Yeast products can contain compounds with immunostimulating properties that are particularly useful for intensive culture operations when animals are in a stressed condition (Li and Gatlin, 2004; McLean and Craig, 2004; Li et al., 2005). Specific products of biological enhancement will have specific benefits, but the cost of producing these products as a fish meal replacement will be the primary factor affecting their use in future aquafeed formulations.
Solid substrate fermentation
Removal of moisture during the production of a feed pellet is energy intensive and one of the most costly steps in the process (Hardy and Barrows, 2000). The same principles apply during biological modification. Instead of fermentations taking place in an aqueous state as with some yeast and bacterial fermentations, solid substrate fermentation (SSF) uses selected strains of fungus to modify substrates in a moist environment. Additional moisture is added continuously to prevent dehydration, since the culture itself heats and tends to dry out. SSF is used commercially to produce a variety of compounds including industrial enzymes, insecticides, and enzyme supplements for the animal feed industry.
Allzyme® SSF (Alltech Inc.) is produced by SSF and expresses at least seven different enzyme activities, including amylase, cellulose, phytase, xylanase, ß-glucanase and others. This product is designed to be a supplement to complete feeds. Dietary supplementation with enzymes has been shown to increase available energy (AMEn), weight gain and feed conversion ratios of broiler chickens (Kocher et al., 2003; Choct et al., 2004).
A Cooperative Research and Development Agreement was developed for a project between the USDA/Agricultural Research Service and Montana Microbial Products, to evaluate the potential of SSF to produce a protein source for aquaculture feeds. Various strains of fungus and substrates were evaluated. Many products were produced and concentrates with protein levels as high as 62% were achieved. ADCs for protein, lipid, and energy for some products for rainbow trout were very high: 97.4%, 98.3%, and 96.7%, respectively. These values are as high as or higher than those for anchovy meal.
Depending on fermentor conditions, however, palatability of the product was affected and feed intake was reduced. Studies are underway to re-evaluate processing conditions and fungal strains to enhance feed consumption. This process may provide a direct method to turn nutrients that are unavailable to trout, or even detrimental, into valuable protein and energy.
Biofuels and aquafeed ingredients
The impact of the growing biofuels industry on animal feeding is somewhat unclear and the subject of many trade and popular press articles. A few aspects of the increased demand for plant materials on the salmonid feed industry are clear. The ethanol industry is based upon starch utilization, and trout and salmon do not readily utilize starch as an energy source. There is no direct competition between salmonid aquaculture and the ethanol industry for starch.
The primary by-product/co-product of this process is distillers dried grains with solubles (DDGS), which has been used effectively in some segments of the animal feeding industry for decades. Most of the current DDGS products are of limited value to salmonids due to the high levels of NSPs. Increasing the content of corn DDGS and corn gluten meal in extruded diets fed to rainbow trout resulted in decreasing weight gains (Stone et al., 2005).
The ADCs, for organic matter and energy in the diet also decreased as the level of corn gluten meal and corn DDGS increased. Increasing availability of DDGS, in its current form, will not benefit salmonid aquaculture. If processing methods can be developed that separate the protein from the NSP, then the improved DDGS will be more valuable for carnivorous fish.
The production of bio-diesel, a replacement for conventional petroleum diesel fuel, is also increasing rapidly along with ethanol production. Soybean oil is currently produced by hexane extraction of soybeans, and the resulting meal used in animal feeds. The valuable extracted oil is used primarily in human foods, but it is increasingly being used as a feedstock for production of bio-diesel.
A new method for soybean meal processing has been developed, with the goal of making soy oil a more economical source for the production of bio-diesel. This method, ‘in situ transesterification,’ eliminates hexane extraction to remove the oil, combining the extraction and transesterification steps into one process for bio-diesel production (Haas et al., 2004). If the resulting meal is comparable in nutritional value to commercially available solvent-extracted soybean meal (SE-SBM), the new process could become widely used in the bio-diesel industry.
A 9-week feeding study was conducted to evaluate the nutritional value of soybean produced by in situ transesterification to rainbow trout. Two levels of each of three types of soybean meal were fed to triplicate lots of 30 (initial wt 22 g) rainbow trout in flow-through 15 oC spring water. The three types of soybean meal included SE-SBM, experimentally produced SE-SBM (ESE-SBM), and meal produced using in situ transesterfication (IS-SBM). Growth rate of fish fed any of the diets was good, averaging over 600% gain (Table 1). There was no effect of type of soybean meal on weight gain.
The trout fed meal processed by the new method (IS-SBM) gained as much weight as the fish fed either of the two control meals. The fish fed the diets containing IS-SBM, however, did have higher feed intakes (2.51% BW/day) as compared to fish fed the ESE-SBM or SE-SBM, 2.38 and 2.46% BW/day, respectively. Since growth rate was equal, feed conversion ratios were higher for the fish fed the IS-SBM.
Protein and energy retention values were significantly lower for the fish fed the IS-SBM diets. The presence of large numbers of Gram-positive bacteria in the intestinal tracts of fish from all diet groups was observed, but only minor effects of these bacteria were noted. Typical soybean enteritis was not observed in fish fed any of the experimental diets. Within the conditions of this study, fish fed the soybean meal produced using the new method had growth equal to trout fed SE-SBM, but FCR was not as favorable.
Table 1. The effect of soybean meal type and level on growth efficiency and nutrient retention of rainbow trout.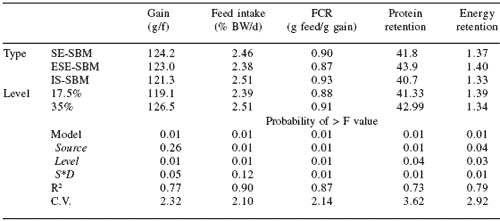
| Conclusion The search for alternative sources of protein to fish meal in salmonid diets has become urgent and intense in recent years. To meet the nutritional needs of salmonids it is likely that future ingredients will be grown or modified to meet specific criteria. Many factors affect the suitability of potential alternatives. The nutritional value, along with the burden of antinutritional and non-nutritional factors and most importantly the cost of the improved characteristics, will ultimately determine their utilization in commercial salmonid diets. |
References
Authors: FREDERIC T. BARROWS and T. GIBSON GAYLORDAdelizi, P.D., R.R. Rosati, K. Warner, Y.V. Wu, T.R. Muench, M.R. White and P.B. Brown. 1998. Evaluation of fish-meal free diets for rainbow trout, Onchorynchus myskiss. Aqua. Nutr. 4:255-266.
Barrows, F.T. and R.W. Hardy. 2001. Nutrition and Feeding. In: Fish Hatchery Management, 2nd Ed. (G. Wedemeyer, ed). John Wiley & Sons, Inc., New York, NY, USA, pp. 483-558.
Barrows, F.T., T.G. Gaylord, D.A.J. Stone and C.E. Smith. 2007. Effect of protein source and nutrient density on growth efficiency of rainbow trout (Oncorhynchus mykiss). Aqua. Res. (in review).
Bairagi, A., K.S. Gosh, S.K. Sen and A.K. Ray. 2004. Evaluation of the nutritive value of Leucaena leucocephala leaf meal, inoculated with fish intestinal bacteria Bacillus subtillis and Bacillus circulans in formulated diets for roho, Labeo rohita (Hamilton) fingerlings. Aqua. Res. 35:436-446.
Cho, C.Y., H.S. Bayley and S.J. Slinger. 1974. Partial replacement of herring meal with soybean meal and other changes in a diet for rainbow trout (Salmo gairdneri). Journal of Fisheries Research Board of Canada 31:1523-1528.
Cho C.Y. and S.J. Slinger. 1979. Apparent digestibility measurement in feedstuffs for rainbow trout. In: Proceedings of the World Symposium on Finfish Nutrition and Fishfeed Technology. Hamburg, Germany, Vol. II, pp. 239-247.
Choct, M., A. Kocher, D.L.E. Waters, D. Peterson and G. Ross. 2004. A comparison of three xylanases on the nutritive value of two wheats for broiler chickens. Brit. J. Nutr. 92:53-61.
Dabrowska, H. and T. Wojno. 1977. Studies on the utilization by rainbow trout (Salmo Gairdneri Rich.) of feed mixtures containing soya bean meal and an addition of amino acids. Aquaculture 10:297-310.
Gatlin III, D.M., F.T. Barrows, P. Brown, J. Campen, K. Dabrowski, T.G. Gaylord, R.W. Hardy, E. Herman, G. Hu, Å. Krogdahl, R. Nelson, K. Overturf, M. Rust, W. Sealey, D. Skonberg, E. Souza, D.A.J. Stone, R. Wilson and E. Wurtele. 2006. Expanding the utilization of sustainable plant products in aquafeeds – A review. Aqua. Res. (in press).
Gaylord,T.G., A.M. Teague and F.T. Barrows. 2006. Taurine supplementation of allplant protein diets for rainbow trout (Oncorhynchus mykiss). J. World Aqua. Soc. 37(4):509-517.
Haas, M.J., K.M. Scott, W.N. Marmer and T.A. Foglia. 2004. In situ alkaline transesterification: an effective method for the production of fatty acid esters from vegetable oils. J. Amer. Oil Chem. Soc. 81(1):83-89.
Hardy R.W. and F.T. Barrows. 2000. Diet formulation and manufacturing. In: Fish Nutrition, 3rd Ed (J.E. Halver and R.W. Hardy, eds). Academic Press Inc., New York, NY, USA, pp. 505-600.
Higgs, D.A., J.R. Markert, D.W. Macquarrie, J.R. McBrie, B.S. Dosanjh, C. Nichols and G. Hoskins. 1978. Development of practical dry diets for coho salmon, Oncorhynchus kisutch, using poultry-by-product meal, feather meal, soybean meal and rapeseed meal as major protein sources. In: Proc. World Symp. on Finfish Nutrition and Fishfeed Technology, Vol. II. Hamburg, Germany, June 20-23, pp. 191-218.
Kaushik, S. J., J.P. Cravedi, J.P. Lalles, J. Sumpter, B. Fauconneau and M. Laroche. 1995. Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic effects, cholesterolemia and flesh quality in rainbow trout. Aquaculture 133:257-274.
Ketola, H.G. and B.F. Harland. 1993. Influence of phosphorus in rainbow trout diets on phosphorus discharges in effluent water. Trans. Amer. Fisheries Soc. 122:1120-1126.
Kocher, A., M. Choct, G. Ross, J. Broz and T.K. Chung. 2003. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 12:275-283.
Lee, K.J., K. Dabrowski, J.H. Blom, S.C. Bai and P.C. Stromberg. 2002. A mixture of cottonseed meal, soybean meal and animal by-product mixture as a fish meal substitute: growth and tissue gossypol enantiomer in juvenile rainbow trout (Oncorhynchus mykiss). J. Anim. Physiol. Anim. Nutr. 86:201-213.
Li, P., G.S. Burr, J. Goff, K.W. Whiteman, K.B. Davis, R.R. Vega, W.H. Neill and D.M. Gatlin III. 2005. A preliminary study on the effects of dietary supplementation of brewers yeast and nucleotides, singularly or in combination, on juvenile red drum, Sciaenops ocellatus. Aqua. Res. 36:1120-1127.
Li, P. and D.M. Gatlin III. 2004. Dietary brewers yeast and the prebiotic GrobioticTM AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops x M. saxatilis) to Streptococcus iniae infection. Aquaculture 231:445-456.
Lindsay, G.J.H. and J.E. Harris. 1980. Carboxymethylcellulase activity in digestive tracts of fishes. J. Fish Bio. 16:219-233.
McLean, E. and S.R. Craig. 2004. Growth performance of Nile tilapia fed and organically certified yeast-based alternative protein source. In: Proceedings of the 5th International Conference on Recirculating Aquaculture (G.J. Flick, A. Correa, T. Rakestraw, eds). Roanoke, VA, USA, July 24-27, pp. 580-586.
McLean, E. and S.R. Craig. 2006. Sustainable feeds for cobia aquaculture: case studies with organically certifiable protein sources. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 22nd Annual Symposium (T.P. Lyons, K.A. Jacques and J.M. Hower, eds). Nottingham University Press, UK, pp. 433-444.
Mukhopadhyay, N. and A.K. Ray. 1999. Effect of fermentation on the nutritive value of sesame seed meal in the diets for rohu, Labeo rohita (Hamilton), fingerlings. Aqua. Nutr. 5:229-236.
Ng, W., H. Lim, S. Lim and C. Ibrahim. 2002. Nutritive value of palm kernel meal pretreated with enzyme or fermented with Trichoderma kongii (Oudemans) as a dietary ingredient for red hybrid tilapia (Oreochromis sp.). Aqua. Res. 33:1199-1207.
Overturf, K., V. Raboy, Z.J. Cheng and R.W. Hardy. 2003. Mineral availability from barley low phytic acid grains in rainbow trout (Oncorhynchus mykiss) diets. Aqua. Nutr. 9:239-246.
Raboy, V., K.A. Young, J.A. Dorsch and A. Cook. 2001. Genetics and breeding of seed phosphorus and phytic acid. J. Plant Physiol. 158:489-497.
Refstie, S., S. Sahlstrom, E. Brathen, G. Baeverfjord and P. Krogedal. 2005. Lactic acid fermentation eliminates indigestible carbohydrates and anti-nutritional factors in soybean meal for Atlantic Salmon (Salmo salar). Aquaculture 246:331-345.
Skrede, G., T. Storebakken, A. Skrede, S. Sahlstrom, M. Sorensen, K.D. Shearer and E. Slinde. 2002. Lactic acid fermentation of wheat and barley whole meal flours improves digestibility of nutrients and energy in Atlantic Salmon (Salmo salar L.) diets. Aquaculture 210:305-321.
Stickney, R.R. and S.E. Shumway. 1974. Occurrence of cellulase activity in the stomach of fishes. J. Fish Bio. 6:779-790.
Stone, D.A.J., R.W. Hardy, F.T. Barrows and Z.J. Cheng. 2005. Effects of extrusion on nutritional value of diets containing corn gluten meal and corn distiller’s dried grain for rainbow trout, Oncorhynchus mykiss. J. Appl. Aqua. 17(3):1-20.
Sugiura, S.H., V. Raboy, K.A. Young, F.M. Dong and R.W. Hardy. 1999. Availability of phoshorus and trace elements in low phytate varieties of barley and corn for rainbow trout (Oncorhynchus mykiss). Aquaculture 170:285-296.
Wilcox, J., G.S. Premachandra, K.A. Young and V. Rayboy. 2000. Isolation of high seed inorganic P, low phytic acid soybean mutants. Crop Sci. 40:1601-1605.
Yamamoto, T., T. Shima, H. Furuita and N. Suzuki. 2002. Influence of feeding diets with and without fish meal by hand and by self-feeders on feed intake, growth and nutrient utilization of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 214:289-305.
US Department of Agriculture, Agricultural Research Service, Hagerman Fish Culture Experiment Station, Hagerman, Idaho, USA



.jpg&w=3840&q=75)