Comparison Between Effects of Sinking and Floating Diets on Growth Performance of Nile Tilapia (Oreochromis niloticus)
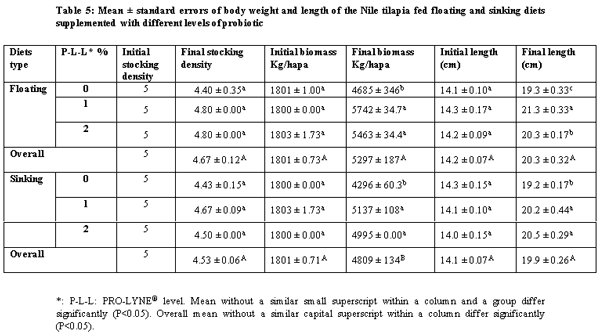
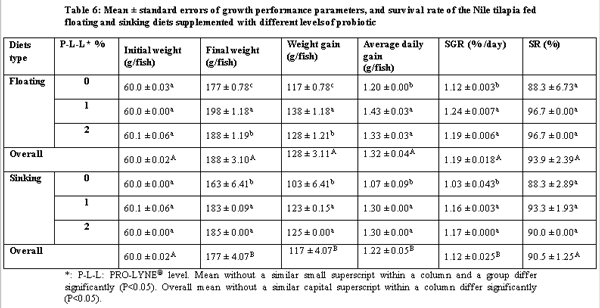
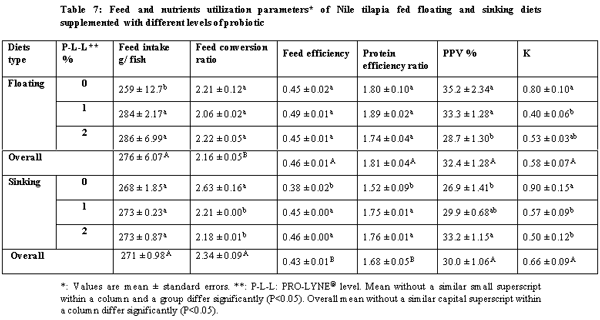
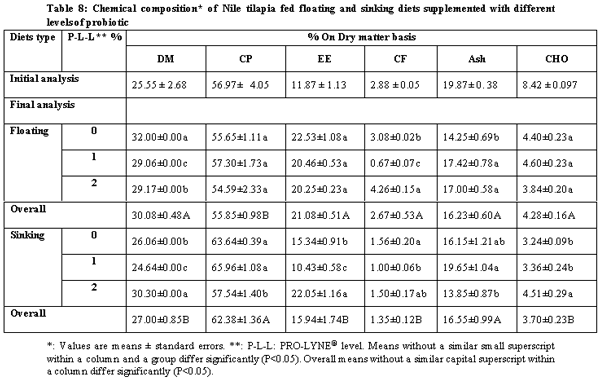
The present study was conducted over 96 days in order to compare between the effects of floating and sinking diets with different levels of a probiotic on growth performance, food utilization and chemical composition with all-male mono-sex juvenile Nile tilapia (Oreochromis niloticus). The treatments were two floating and sinking diets supplemented with three levels of the probiotic PRO-LYNE®, being 0%, 1% and 2% of the diets. Each hapa (measuring 2 × 3 × 1 m3) was suspended in an earthen pond (4000 m2). There were 6 treatments, each consisting of 2 replicates, stocked with fish of average initial body weigh 60 g. A total of 366 fish were randomly distributed into 6 experimental groups. The stocking density was 5 fish / m3 for all the hapas. Fingerlings were fed a commercial diet containing 25.2% crude protein and at feeding rates of 4% of their fresh biomass in each hapa for the first 1.5 month and 3% until the end of the experiment. The results of the present study revealed that performance and production and economic efficiency of Nile tilapia reared in net hapas in earthen ponds significantly increased, when they were fed the floating pellets supplemented with probiotic at level 1%.
Keywords: Nile tilapia, floating and sinking diets, probiotic, growth parameters, feed utilization, chemical composition.
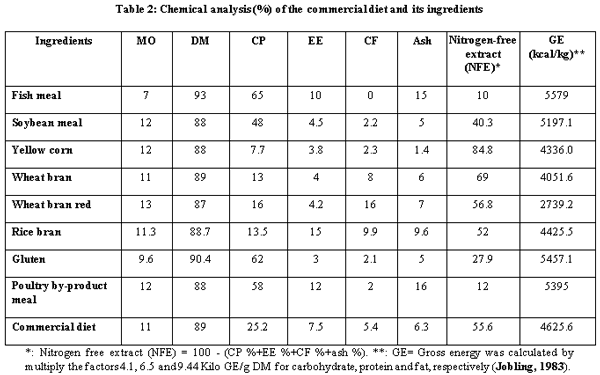
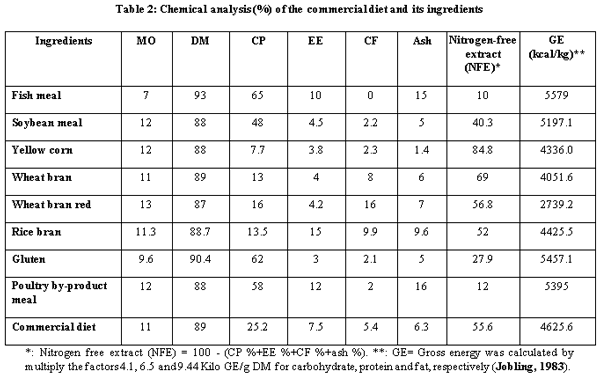
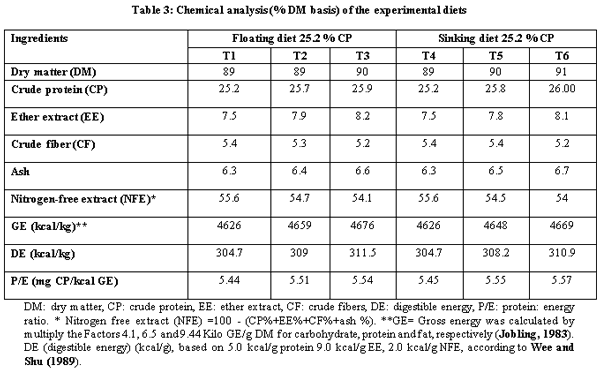
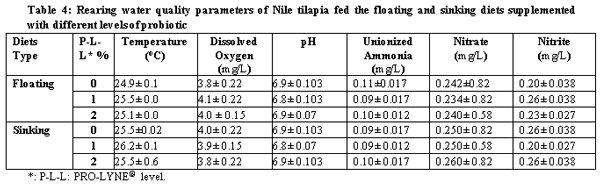
A.O.A.C. (1990). Official Method of Analysis, 15th Ed. Association of Official Analysis of Chemists, Washington D.C.
Abd El-Maksuod, A.M.; Allam, S.M., Abou-Fotoh, G.E. and Abou Zied, R.M. (1998). Evaluation of Nigella seeds as a feed additive in diets of Nile tilapia. Fayoum. J. Agric. Res. & Dev., 12:170-181.
Abdel-Hakim, N.F.; Bakeer, M.N. and Soltan, M.A. (2002). Water Environment for Fish Culture. Deposition No. 4774.
Abdelhamid, A.F.B. (2019a). Water Quality Properties in Fish Farms. 99P, Al-Azhar Univ. (under publication).
Abdelhamid, A.M. (1996). Field and Laboratorial Analysis in Animal Production. Dar Al-Nashr for the Egyptian Universities. Cairo, 680p, 977-5526-47-7, Deposition No. 11318/1996.
Abdelhamid, A.M. (2019b). The Modern Methods of Aquaculture. Deposit No. 25438/2018, 718 P.
Abdelhamid, A.M.; khalil, F.F., El Barbary, M.I., Zaki, V.H. and Hussein, H.S. (2002). Feeding Nile tilapia on Biogen to detoxify aflatoxin diets. Proc. Con. Animal, Fish Prod. Mansoura, 24- 25 Sep., pp: 207-230.
Abdoulaye, L. (2013). Effect of dietary protein level on growth performance carcass composition and survival rate of fry mono-sex Nile tilapia O. niloticus reared under re- circulating system. Journal of Biology and Life Science, 4 (2).
Abou-Zied, R.M. (2015). Effect of diet extruded type on growth performance feed utilization and economic efficiency of Nile tilapia in commercial farms. Egyptian J Nutrition and Feeds 18 (1):143-150.
Ahmed, G.U.; Sultana, N., Shamsuddin, M. and Hossain, M.B. (2013). Growth and production performance of mono-sex tilapia (Oreochromis niloticus) fed with homemade feed in earthen mini ponds. Pakistan J. Biol. Sci., 16 (23):1781-1785.
Ahmed, T.; Hasan, S.J., Hossain, M.R., Haidar, I., Rubel, A.K.M.S.A. and Pramanik, M.H. (2014). Assessment on impact of dietary probiotic supplementation on growth indices of mono-sex tilapia (Oreochromis niloticus) cage culture at Dakatia River, Chandpur, Bangladesh. World Journal of Fish and Marine Sciences, 6 (5): 441-446.
Ahmed, M.; Qureshi, T., Singh, A.B., Susan, N., Kamlesh, B. and Salman, R.C. (2012). Effect of dietary protein, contest growth, feed efficiency and carcass composition of Cyprinus carpio communes fingerlings. International Journal of Fisheries and Aquaculture, 4 (3): 30-40.
Aly, S.M.; Mohamed, M.F. and John, G. (2008). Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquaculture Research, 39: 647–656.
APHA (2005). Standard Methods for the Examination of Water & Wastewater. American Public Health Association.
Boyd, C.E. (1982). Water Quality Management for Pond Fish Culture. Elsevier Scientific Publishing Co. Amsterdam: 318.
Burr, G.; Hume, M., Neill, W.H. and Gatlin III, D.M. (2008). Effects of probiotics on nutrient digestibility of soybean-meal-based diets by red drum (Sciaenops ocellatus). Aquaculture Research, 39: 1680–1686.
Cowey, C.B. and Sargent, J.R. (1979). Nutrition. In: Fish Physiology. (eds. W.S. Hoar, D.J. Randall and J.R. Brett), pp. 1-69. Academic Press, London.
Cruz, P.M.; Ibanez, A.L., Hermosillo, O.A.M. and Ramirez Saad, H.C. (2012). Use of probiotics in aquaculture. International Scholarly Research Network (ISRN), Microbiology, Volume 2012. pp. 13.
Davis, A.T. and Stickney, R.R. (1978). Growth responses of Tilapia aurea to dietary protein quality and quantity. Transaction of the American Fisheries Society, 107: 479-483.
Defoirdt, T.; Sorgeloos, P. and Bossier, P. (2011). Alternatives to antibiotics for the control of bacterial disease in aquaculture. Current Opinion in Microbiology, 14: 251–258.
Diana, J.S.; Lin, C.K. and Yi, Y. (1996). Timing of supplemental feeding for tilapia production. J. World Aquacult. Soc., 27: 410-419.
Duncan, D. (1955). Multiple range and multiple F-tests. Biometrics, 11: 1-42.
El-Rhman, A.M.A.; Khattab, Y.A. and Shalaby, A.M. (2009). Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunology, 27: 175-180.
FAO (Food and Agriculture Organization) (2004). Fishstat Plus. Aquaculture Production 1950- 2002. Food and Agriculture Organization FAO, Rome, Italy.
FAO (Food and Agriculture Organization) (2012). Fishstat Plus, Vers. 2.32. Rome, Italy. Ferguson, R.M.W.; Merrifield, D.L., Harper, G.M., Rawling, M.D. and Mustafa, S. (2010). The effect of Pediococcus acidilactici on the gut microbiota and immune status of on-growing red tilapia (Oreochromis niloticus). Journal of Applied Microbiology, 109: 851-862.
Fitzsimmons, K. (2004). Development of new products and markets for the global tilapia trade. In: Proceeding of the 6th International Symposium on Tilapia in Aquaculture, Manila, Philippines (ed. By R. Bolivar, G. Mair & K Fitzsimmons), pp: 624-633.
Grisdale-Helland, B.; Helland, S.J. and Gatlin, D.M. (2008). The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture, 283: 163-167.
Gumus, E. and Ikiz, R. (2009). Effect of dietary levels of lipid and carbohydrate on performance, chemical contents and digestibility in rainbow trout, Oncorhynchus mykiss. Pakistan Veterinary Journal, 29 (2): 59-63.
Hassan, R. (1992). Acute ammonia toxicity of red tilapia and sea bass. Fisheries Bulletin of the Department of Fisheries, Malaysia No. 73. 8 pp.
Honsheng, M. (2010). Effects of probiotics on activities of digestive enzyme for tilapia. Journal of South China Normal University, 2: 10-15.
Irianto, A. and Austin, B. (2002). Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases, 25: 333–342.
Jauncey, K. (1982). The effects of varying dietary protein on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture, 27: 43-54.
Jobling, S. (1983). A total protein and acid needs. In: I.J.E. Halve, Nutrition. Academic Press: New York, pp: 106-143.
Kannadhason, S.; Muthukumarappan, K. and Rosentrater, K.A. (2009). Effects of ingredients and extrusion parameters on aquafeeds containing DDGS and tapioca starch. J. Aquacult. Feed Sci. Nutr., 1 (1): 6-21.
Kunda, M.; Dev, A.R. and Islam, M.J. (2015). Comparison of production performance and economics between mono-sex and mixed-sex tilapia (Oreochromis niloticus). SAU Res. Prog. Rep. No. 02, Sylhet Agricultural University, Sylhet, Bangladesh, 31-37 pp.
Lim, C.E.; Webster, C.D. and Li, M.H. (2006). Feeding Practices. In: C.E. Lim & C.D. Webster, eds. Tilapia Biology, Culture and Nutrition, pp. 547–559. New York, Haworth Press Inc., 678 pp.
Limbu, S.M. and Jumanne, K. (2014). Effect of restricted and re-feeding regime on feeding cost, growth performance, feed utilization and survival rate of mixed sex Nile tilapia Oreochromis niloticus cultured in tanks. Int. J. Fish Aquat. Stud., 2 (1): 118-123.
Marzouk, M.S.; Moustafa, M.M. and Mohamed, N.M. (2008). The influence of some probiotics on the growth performance and intestinal microbial flora of Oreochromis niloticus. Proceedings of 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt. pp. 1059-1071.
Mazid, M.A.; Tanaka, Y., Katayama, T., Rahman, M.A., Simpson, K.L. and Chichester, C.O. (1979). Growth response to Tilapia zillii fingerlings fed isocaloric diets with variable protein levels. Aquaculture, 18: 115-122.
Merrifield, D.L.; Dimitroglou, A., Foey, A., Davies, S.J. and Baker, R.T.M. (2010). The current status and future focus of probiotic and probiotic applications for salmonids. Aquaculture, 302: 1-18.
Mussatto, S.I. and Mancilha, I.M. (2007). Non-digestible oligosaccharides: A review, Carbohydrate Polymers, 68: 587-597.
NRC (1993). Nutrient Requirement of Fish. National Academy Press. Washington, Dc, P.114.
Nwanna, L.C. (2002). Performance of hybrid Clariid catfish fingerlings (Clarias gariepinus ? × Heterobranchus bidorsalis ?) fed poultry layer waste diets in aquaria. J. Appl. Aquacult., 12 (3): 99-106.
Otte, G. and Rosenthal, H. (1979). Management of a closed brackish water system for high density fish culture by biological and chemical water treatment. Aquaculture, 18: 169-181.
Pedro, N.D.; Guijarro, A.L., Delgado, M.J., Patina, L.P., Pinillos, M.L. and Bedate, M.A. (2001). Influence of dietary composition on growth and energy reserves in Tench, Tincta tincta. Journal of Applied Ichthyology, 17: 25-29.
Pfeffer, E. (1982). Utilization of dietary protein by salmonids. Comparative Biochemistry and Physiology (B), 73: 51-57.
Rahman, M.S. (1992). Water quality management in aquaculture. BRAC Prokashana, 66, Mohakhali, Dhaka-1212, Bangladesh: 84.
Saha, S.B. and Khatun, M.S. (2014). Production performance of mono-sex Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) in brackish water ponds. Bangladesh J. Zool., 42 (2): 261-269.
Santiago, C. B. and Lovell, R.T. (1988). Amino acid requirements for growth of Nile tilapia. The Journal of Nutrition, 118 (12): 1540-1546.
SAS (2006). SAS statistical guide for personal computer, SAS Institute Inc. Cary, NC.
Shelby, R.A.; Lim, C.E., Aksoy, M. and Klesius, P.H. (2006). Effect of probiotic diet supplements on disease resistance and immune response of young Nile tilapia, Oreochromis niloticus. Journal of Applied Aquaculture, 18: 23-34.
Shul’man, G.E. (1974). Life cycles of fish. Physiology and Biochemistry. Keter Publishing House, Jerusalem, 258 pp.
Sinha, A.K; Kumar, V., Makkar, H.P., De Boeck, G. and Becker, K. (2011). Non-starch polysaccharides and their role in fish nutrition–A review. Food Chem., 127 (4): 1409-1426.
Sorensen, M. (2012). A review of the effects of ingredient composition and processing conditions on the physical qualities of extruded high-energy fish feed as measured by prevailing methods. Aquacult. Nutr., 18 (3): 233-248.
Tidwell, J.H.; Coyle, S.D., Bright, L.A. and Asharian, Y. (2005). Evolution of plant and animals source proteins for replication of fish meal in practical diets for the largemouth bass, Microbteus salmoides. Journal World Aquaculture Society, 36: 454-463.
Verschuere, L.; Rombaut, G., Sorgeloos, P. and Verstraete, W. (2000). Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Review, 64: 655-671.
Wang, Y.C.; Chang, P.S. and Chen, H.Y. (2008). Differential time series expression of immune related genes of Pacific white shrimp in response to dietary inclusion of β-1, 3-glucan. Shellfish Immunology, 24: 113-121.
Wee, K.L. and Shu, S.W. (1989). The nutritive value of boiled feed –fat Soy bean in pelted feed for Nile tilapia. Aquaculture, 81:303-314.
Wilson, R.P.; Gatlin III, D.M. and Poe, W.E. (1985). Postprandial changes in serum amino acids of channel catfish diets containing different levels of protein and energy. Aquaculture, 49: 101-110.
Winfree, R.A. and Stickney, R.R. (1981). Effects of dietary protein and energy on growth, feed conversion efficiency and body composition of Tilapia aurea. Journal of Nutrition, 111: 1001-1012.
Yamamoto, T.; Unuma, T. and Akiyama, T. (2000). The influence of dietary protein and fat levels on tissue free amino acid levels of fingerling rainbow trout (Oncorhynchus mykiss). Journal of Aquaculture, 182 (3-4): 353-372.
Yousefian, M. and Amiri, M.S. (2009). A review of the use of probiotic in aquaculture for fish and shrimp. African Journal of Biotechnology, 8: 7313-7318.
Zhou, Z.G.; Ding, Z.K. and Huiyuan, L.V. (2007). Effects of dietary short-chain fructooligosaccharides on intestinal microflora, survival, and growth performance of juvenile white shrimp, Litopenaeus vannamei. Journal of the World Aquaculture Society, 38: 296–301.





.jpg&w=3840&q=75)