A Bacillus probiont for Black Tiger shrimp culture
As in animal husbandry, prevention is always better than cure, and prevention of disease in aquaculture requires the creation and maintenance of biosecure environmental conditions and the enhancement of innate immune response in the animals cultivated. The use of probiotics as a means of reducing disease based on the principle of competitive exclusion and the application of immunomodulating compounds that enhance the innate immune response of shrimp, are two of the most promising preventive methods developed during the last few years.
Unlike vertebrates, including fish, shrimp do not possess immunoglobulins and do not appear to develop acquired immunity (Sritunyalucksana and Söderhäll 2000; Vargas-Albores and Yepiz-Plascencia, 2000); therefore vaccination seems to be impossible. Arthropods, including shrimp and other crustaceans, appear to depend upon the non-specific immune system. Several recognition molecules such as ß-1,3-glucan binding protein (BGBP), lipopolysaccharide (LPS)- binding protein and peptidoglycan-binding protein (PGBP) isolated from plasma of arthropods, horseshoe crabs, and freshwater crayfish have been shown to induce activation of the prophenoloxidase (proPO) system in shrimp as well as stimulate other defensive cellular functions such as phagocytosis, melanization, encapsulation and coagulation. Shrimp haemolymph contains these recognition molecules as well as haemocyanin (the principal oxygen-carrying molecule) and circulating haemocytes (granulocytes and phagocytic cells). The BGBP reacts with ß-glucans from invading pathogens and the resulting glucan-BGBP complex induces degranulation of the granulocytes and the activation of the proPO system. A similar reaction also occurs in the case of LPS and PGBP when these are present.
Once an infection is over, the immune cells lack any specific immune ‘memory’ of the infectious agent and there are no circulating antibodies that would allow a more rapid, specific response to subsequent exposure or infection. Detailed understanding of the immune responses of crustaceans is still lacking and extensive studies are still required if we are to develop a greater knowledge of shrimp immunity. However, it is expected that it will be possible in the near future to combine such knowledge of shrimp defense mechanisms to better control diseases.
By comparison with the knowledge of the shrimp immune system, the use of probiotics in aquaculture has been more intensively researched and documented due to their importance in limiting the occurrence of pathogenic bacteria in hatcheries and grow-out ponds. This paper will review some of the research that has been conducted in Thailand to develop a probiotic specifically for shrimp culture.
Probiotics and immunostimulants
PROBIOTICS
There is confusion in the aquaculture literature as to the application of the term ‘probiotic’ with respect to the method of administration. The term probiotic has been used to include bacteria, or mixtures of bacteria, added directly to the water to influence the microbial environment of the culture system (more properly referred to as bacterial amendments), as well as bacteria added through the feed to colonise the gut and improve feed utilization and animal health through the action of beneficial microorganisms in the gastrointestinal tract of the host. In this paper, a strict definition of probiotic will be used, i.e. microbial cells that are administered in such a way as to enter the gastrointestinal tract and to be kept alive, as suggested by Gatesoupe (1999), or a live microbial feed supplement that beneficially affects the host animal by improving intestinal microbial balance (Fuller, 1989).
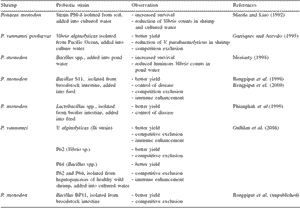
Bacteria that have been used successfully as probiotics at the level of laboratory and grow-out ponds for shrimp culture generally belong to the genera Bacillus, Lactobacillus, and Vibrio (Table 1). Most of these probiotics were isolated from the culture system or related areas (for example water, soil or the host intestine) and generally applied as a monoculture added into either pond water or feed.
The principal mode of action for probiotic bacteria is thought to be through competitive exclusion mechanisms in which pathogens are replaced or excluded through the development of a beneficial microbial population. This replacement or exclusion of pathogens on the intestinal surface by probiotics then leads to a reduction in disease and better health in the host.
Increased immunity and disease resistance of the Black Tiger shrimp, Penaeus monodon, against Vibrio harveyi, an important pathogen of shrimp in commercial culture, has been shown following administration of the Bacillus S11 probiont in the feed (Rengpipat et al., 2000).
Bacillus S11 (Rengpipat et al., 1998), identified as a strain of Bacillus subtilis, was originally isolated from the intestine of healthy Thai shrimp broodstock. Direct addition of Bacillus S11 into feed given to P. monodon resulted in greater live weight gain and survival of shrimp in the probiotic group (Figure 1). The probiont treatment group mean weight (7.06 ± 0.48 g) was also greater (P>0.05) than that of the control group (3.99 ± 0.38 g).
Table 1. Probiotics used in shrimp culture.
To enlarge the image, click here
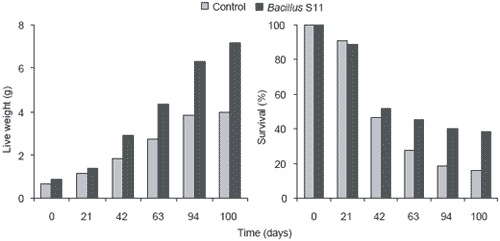
Figure 1.Live weights (g) and survival (%) of Penaeus monodon cultured for 100 days in Bacillus S11 and control treatments.
The effect of the probiotic addition on the shrimp’s ability to withstand a disease challenge was then investigated. After a 10-day challenge with V. harveyi, a causative agent for luminescent disease in shrimp, all shrimp in the probiotic group survived compared to only 26% of shrimp in the control group given regular feed (Figure 2). The colonization of the shrimp gut by Bacillus S11 presumably protected P. monodon against pathogenic bacterial infection through competitive exclusion. Gullian et al. (2004) also reported that P. vannamei immersed in a suspension of Bacillus strain P64 in cultured water, showed better growth and higher disease resistance than the control shrimp. Although the delivery route was different (immersion vs. oral), both Bacillus probiotics enhanced shrimp growth and disease resistance in different species of Penaeid (Table 2).
IMMUNOSTIMULANTS
An immunostimulant is any substance that is intended to boost the immune response and enhance resistance to microbial pathogens. Smith et al. (2003) classified immunostimulants claimed to increase survival of crustaceans following experimental exposure to infectious microorganisms, into five kinds: (i) live bacteria; (ii) killed bacteria (bacterins or bacterial antigen); (iii) glucans; (iv) peptidoglycans; and (v) lipopolysaccharides (LPS).
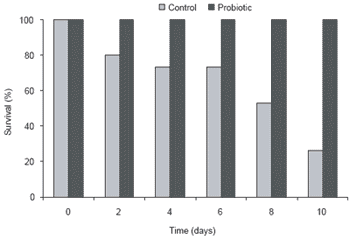
Figure 2. Effect of probiotic treatment on Penaeus monodon survival after challenge with Vibrio harveyi for 10 days.
Table 2. Probiotics and their effects on shrimp health and immunity indices.

To enlarge the image, click here
The use of live and/or killed bacteria of non-virulent or attenuated pathogens, similar to traditional vaccination, has been attempted. Resistance to vibriosis in P. monodon treated with formalin-killed selected Vibrio spp. has been reported (Teunissen et al., 1998), as has resistance in P. indicus larvae exposed to freezedried V. harveyi by immersion (Alabi et al., 1999). Alabi et al. (2000) also detected antibacterial activity in the haemolymph of P. vannamei exposed to live, nonpathogenic V. harveyi strain DPEX.
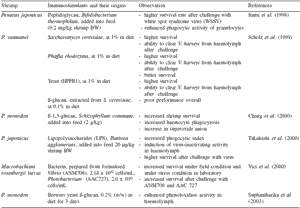
Immune stimulants based on peptidoglycan, glucan, and LPS from the cell walls of non-pathogenic bacteria or fungi (Table 3) also showed enhancement of disease resistance against Vibrio infection and increased immunity indices. The defense responses elicited by BS11 in shrimp in our laboratory studies are also shown in Table 4 (Rengpipat et al., 2000). The values for total haemocyte count, phagocytic activity, phenoloxidase and antibacterial activity were all greater in probioticfed shrimp than in the control groups. Furthermore, after 10 days’ challenge with V. harveyi, all of the immune parameters of probiotic shrimp were greater (P<0.05) than the controls (Table 5). Following the challenge, all of the immune parameters in both control and probiotic treatments increased substantially, with the exception of total haemocyte count, which decreased.
Table 3. Immunostimulants in shrimp culture.
To enlarge the image, click here
It appears that viable cells of BS11 colonise the shrimp gut and their antigens, whether dead cells, components of digested cells such as peptidoglycans, or other, as yet unknown, antigens, may play an important role to promote the immune defense response in shrimp.
Field trials with Bacillus S11 Bacillus S11 was used as a supplement in feed for Black Tiger shrimp in two earthen pond field trials in Thailand. The trials were conducted over 100 days during two different seasons, and growth and survival were compared with those of shrimp receiving an unsupplemented feed. In both seasons, shrimp fed the probiotic feed grew significantly larger and had higher survival than shrimp fed unsupplemented feed (P<0.05) (Figure 3) (Rengpipat et al., 2003). This indicates that Bacillus S11 has considerable potential for use as probiotic supplement for feed in the commercial culture of black tiger shrimp.
Conclusion
Apart from sound management of the pond environment to maintain good conditions for commercial shrimp culture, routine prophylaxis for shrimp should be considered since shrimp often face stressful conditions even in the best-managed farms. Over-reliance on chemical and antibiotic use in the past has been counterproductive as well as having a negative impact on public perceptions of the food safety of farmed shrimp. Both probiotics and immunostimulants are good candidates to replace the use of chemicals and antibiotics in the development of sustainable shrimp farming systems. Bacillus spp. appear to be good probionts as well as showing potential as immunostimulants. The ongoing search for new probiotic strains and further experimentation on interactions between immunostimulant and shrimp immunity at the molecular level will shed more light on how to control diseases in shrimp culture.
Table 4. Mean immunity index values of control- and probiotic- treated Penaeus monodon during 90 days of culture.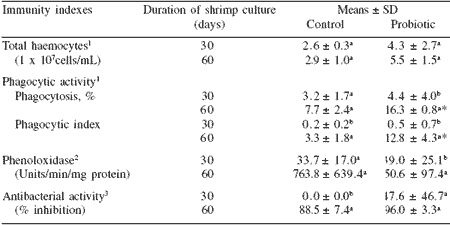
1n = 3; 2n = 5; 3n = 6
abMeans in a column (for each index) not sharing a common superscript letter differ (P<0.05)
*Indicates difference (P<0.05) between control and probiotic treatment means
Shrimp age started at ~PL-60
Modified from Rengpipat et al., 2003
Table 5. Immunity indices in Penaeus monodon between control and probiotic groups after 10 days challenge with Vibrio harveyi .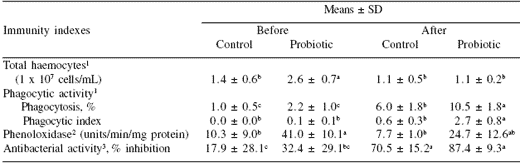
1 n = 3; 2 n = 5; 3 n = 6
abMeans in a row not sharing a common superscript differ (P<0.05)
Shrimp age started at ~PL-10, cultured for 100 days before challenge test
Modified from Rengpipat et al., 2003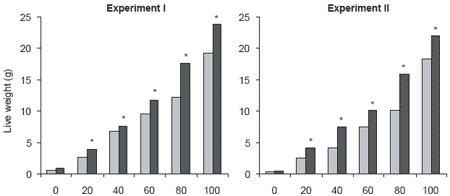
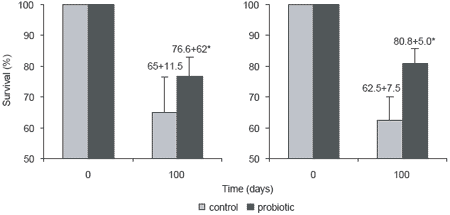
Figure 3. Average weights and survivals of Penaeus monodon during 100 days of culture when given either a control diet or one supplemented with probiotic (*Means differ, P<0.05).
Acknowledgements
The author is grateful to Dr. Daniel F. Fegan for reviewing the manuscript. This research was supported by the Thailand Research Fund.
References
Alabi, A.O., J.W. Latchford and D.A. Jones. 1999. The efficacy of immersion as opposed to oral vaccination of Penaeus indicus larvae against Vibrio harveyi. Aquaculture 178:1-11.
Alabi, A.O., J.W. Latchford and D.A. Jones. 2000. Demonstration of residual antiactivity in plasma of vaccinated Penaeus vannamei. Aqauculture 187:15- 34.
Chang, C.F., H.Y. Chen, M.S. Su and I.C. Liao. 2000. Immunomodulation by dietary ß-1,3-glucan in the brooders of the Black Tiger shrimp, Penaeus monodon. Fish Shellfish Immunol. 10:505-514.
Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378.
Garriques, D. and G. Arevalo. 1995. An evaluation of the production and use of a live bacterial isolate to manipulate the microbial flora in the commercial production of Penaeus vannamei postlarvae in Ecuador. In: Swimming through Troubled Water, Proceedings of the Special Session on Shrimp Farming, Aquaculture, 95 (C.L. Browdy and J.S. Hopskins, eds). World Aquaculture Society, Baton Rouge, LA, USA, pp. 53-59.
Gatesoupe, F.J. 1999. The use of probiotics in aquaculture. Aquaculture 180:147-165.
Gullian, M., F. Thompson and J. Rogriguez. 2004. Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture 233:1-14.
Itami, T., M. Asano, K. Tokushige, K. Kubono, A. Nakagawa, N. Takeno, H. Nishimura, M. Maeda, M. Kondo and Y. Takahashi. 1998. Enhancement of disease resistance of Kuruma shrimp, Penaeus japonicus, after oral administration of peptidoglycan derived from Bifidobacterium thermophilum. Aquaculture 164:277-288.
Maeda, M. and I.C. Liao. 1992. Effect of bacterial population on the growth of prawn larva, Penaeus monodon. Bull. Natl. Res. Inst. Aquacult. 21:25-29.
Moriarty, D. 1998. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 164:351- 358.
Phianphak, W., S. Rengpipat, S. Piyatiratitivorakul and P. Menasveta. 1999. Probiotic use of Lactobacillus spp. for Black Tiger shrimp, Penaeus monodon. J. Sci. Res. Chula. Univ. 24:41-51.
Rengpipat, S., S. Rukpratanporn. S. Piyatiratitivorakul and P. Menasveta. 2000. Immunity enhancement in Black Tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 191:271-288.
Rengpipat, S., W. Phianphak, S. Piyatiratitivorakul and P. Menasveta. 1998. Effect of probiotics on Black Tiger shrimp, Penaeus monodon, survival and growth. Aquaculture 167:301-313.
Rengpipat, S., A. Tunyanun, A.W. Fast, S. Piyatiratitivorakul and P. Menasveta. 2003. Enhanced growth and resistance to Vibrio challenge in pondreared Black Tiger shrimp, Penaeus monodon, fed a Bacillus probiotic. Dis. Aqua. Org. 55:169-173.
Scholz, U., G. Garcia-Diaz, D. Ricque, L.E. Cruz- Suarez, F. Vargas-Albores and J. Latchford. 1999. Enhancement of vibriosis resistance in juvenile Penaeus vannamei by supplementation of diets with different yeast products. Aquaculture 176:271-283.
Smith, V.J., J.H. Brown and C. Hauton. 2003. Immunostimulation in crustaceans: does it really protect against infection? Fish & Shellfish Immunol. 15:71-90.
Sritunyalucksana, K. and K. Söderhäll. 2000. The proPO and clotting system in crustaceans. Aquaculture 191:53-69.
Suphantharika, M., P. Khunrae, P. Thanardkit and C. Verduyn. 2003. Preparation of spent brewer’s yeast ß-glucans with a potential application as an immunostimulant for Black Tiger shrimp, Penaeus monodon. Bioresource Tech. 88:55-60.
Takahashi, Y., M. Kondo, T. Itami, T. Honda, H. Inagawa, T. Nishizawa, G.I. Soma and Y. Yokomizo. 2000. Enhancement of disease resistance against penaeid acute viraemia and induction of virusinactivating activity in haemolymph of Kuruma shrimp, Penaeus japonicus, by oral administration of Pantoea agglomerans lipopolysaccharide (LPS). Fish Shellfish Immunol. 10:555-558.
Teunissen, O.S.P., R. Faber, G.H.R. Booms, T. Latscha and J.H. Boon. 1998. Influence of vaccination on vibriosis resistance of the giant Black Tiger shrimp Penaeus monodon (Fabricius). Aquaculture 164:359- 366.
Vargas-Albores, F. and G. Yepiz-Plascencia. 2000. Beta glucan binding protein and its role in shrimp immune response. Aquaculture 19:13-21.
Vici, V., I.S. Bright Singh and S.G. Bhat. 2000. Application of bacterins and yeast Acremonium dyosporii to protect the larvae of Macrobrachium rosenbergii from vibriosis. Fish Shell Immunol. 10:559-563.
Author: SIRIRAT RENGPIPAT
Department of Microbiology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand


