The first evidence of a new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and unvaccinated broiler flocks in Algeria
Background and Aim: Avian infectious bronchitis virus (IBV) frequently infects broilers and is responsible for severe economic losses to the poultry industry worldwide. It has also been associated with kidney damage in the broiler flocks. The aim of the present study is to determine the presence of IBV and its possible involvement in kidney damage of broiler chicks.
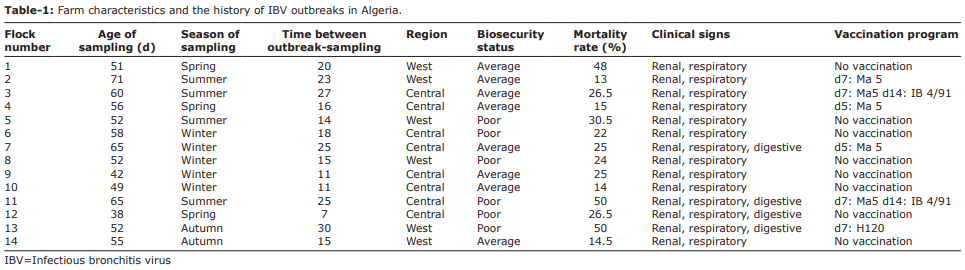
Materials and Methods: 14 clinically diseased broiler flocks from Western and Central Algeria were sampled and analyzed by hemagglutination inhibition (HI) test and reverse transcriptase-polymerase chain reaction (RT-PCR) followed by phylogenic analysis.
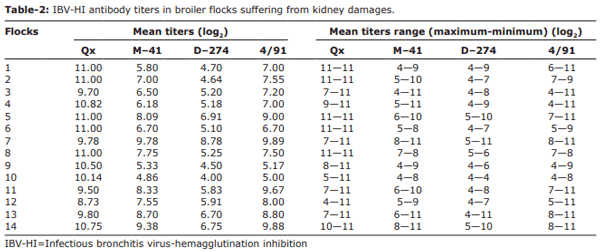
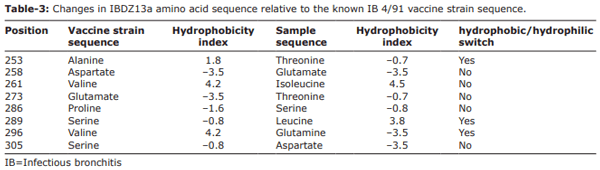
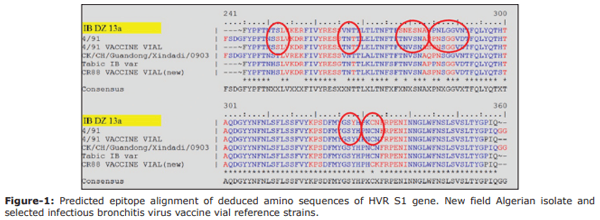
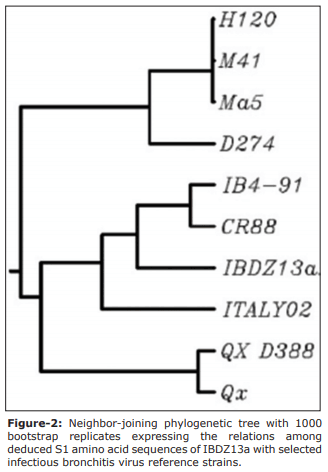
Results: The QX (100%) and 4/91 (60%) IBV serotypes were the most prevalent in the kidney damaged broilers regardless of vaccination status. The molecular detection of avian IBV by RT-PCR identified six samples as positive, of which only two isolates were typable by sequencing. We identified a novel IBDZ13a genotype which showed 93% sequence homology to the partial-S1 gene sequence of the IB 4/91 commercial vaccine strain. Sequencing analysis characterized this virus as a novel and divergent IB 4/91 field virus with eight amino acid substitutions that might have resulted in altered immunogenicity.
Conclusion: The isolation of a new IBV strain (IBDZ13a) from vaccinated broiler flocks may explain the failure of the vaccination programs against IBV field strains. Combination of the HI test and RT-PCR indicated that the nephropathogenic IB outbreaks in broilers are related to this novel strain.
Keywords: avian infectious bronchitis virus, broilers, kidney damages, Algeria, hemagglutination inhibition test, reverse transcriptase-polymerase chain reaction, IBDZ13a.
1. Cavanagh, D. and Gelb, J.J. (2008) Infectious bronchitis. In: Diseases of Poultry. 12th ed. Blackwell Publishing, Iowa. USA. p117-135.
2. Schalk, A.F. and Hawn, M.C. (1931) An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. As., 78: 413-416.
3. Shengwang, L. and Kong, X. (2004) A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol., 33(3): 321-327.
4. Winterfield, R.W. and Hitchner, S.B. (1962) Etiology of an infectious nephritis-nephrosis syndrome of chickens. Am. J. Vet. Res., 23(11): 1273-1279.
5. Zanella, A., Lavazza, A., Marchi, R., Martin, A.M. and Paganelli,F. (2003) Avian infectious bronchitis: Characterization of new isolates from Italy. Avian Dis., 47(1): 180-185.
6. Cuiping, X., Jixun, Z. and Xudong, Z. (2007) Isolation and identification of four isolates of infectious bronchitis strains in China and analysis of their SI protein gene. Vet. Microbiol., 122(1-2): 61-71.
7. Shimazaki, Y., Horiuchi, T., Harada, M., Tanimura, C., Seki, Y., Kuroda, Y., Yagyu, K., Nakamura, S. and Suzuki, S. (2008) Isolation of 4/91 type of infectious bronchitis virus as a new variant in Japan and efficacy of vaccination against 4/91 type field isolate. Avian Dis., 52(4): 618-622.
8. Awad, F., Chhabra, R., Baylis, M. and Ganapathy, K. (2014) An overview of infectious bronchitis virus in chickens. Worlds Poult. Sci. J., 70(2): 375-384.
9. Fellahi, S., Harrak, M.E.L., Ducatez, M., Saad Ibn Souda Koraichi, L., Kuhn, J.H., Khayi, S., Houadfi, M.L., Ennaji, M.M. (2015) Phylogenetic analysis of avian infectious bronchitis virus S1 glycoprotein regions reveals emergence of a new genotype in Moroccan broiler chicken flocks. Virol. J., 12(1): 116.
10. Abdel-Moneim, A.S., El-Kady, M.F., Ladman, B.S. and Gelb, J. (2006) S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J., 3:78.
11. Cavanagh, D., Picault, J.P., Gough, R.E., Hess, M., Mawditt, K. and Britton, P. (2005) Variation in the spike protein of the 793/B type of infectious bronchitis virus, in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol., 34(1): 20-25.
12. Li, L., Xue, C., Chen, F., Qin, J., Xie, Q. and Bi, Y. (2010) Isolation and genetic analysis revealed no predominant new strains of avian infectious bronchitis virus circulating in South China during 2004-2008. Vet. Microbiol., 143(2-4): 145-154.
13. Worthington, K.J., Currie, R.J.W. and Jones, R.C. (2008) A reverse transcriptase polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol., 37(2): 247-257.
14. Bochkov, Y.A., Batchenko, G.V., Shcherbakova, L.O., Borisov, A.V. and Drygin, V.V. (2006) Molecular epizootiology of avian infectious bronchitis virus in Russia. Avian Pathol., 35(5): 379-393.
15. Cavanagh, D. (2007) Coronavirus avian infectious bronchitis virus. Vet Res., 38(2): 281-297.
16. Wang, L., Junker, D. and Collisson, E.W. (1993) Evidence of natural recombination within the S1 gene of infectious bronchitis virus. Virol. J., 192(2): 710-716.
17. Gelb, J., Weisman, Y., Ladman, B.S. and Meir, R. (2005) Gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000). Avian Pathol., 34(3): 194-203.
18. Xu, G., Liu, X., Zhao, Y., Chen, Y., Zhao, J. and Zhang, G. (2016) Characterization and analysis of an infectious bronchitis virus strain isolated from southern China in 2013. Virol. J., 13:40.
19. El Bouqdaoui, M., Mhand, R.A., Bouayoune, H. and Ennaji, M.M. (2005) Genetic grouping of nephropathogenic avian infectious bronchitis virus isolated in Morocco. Int. J. Poult. Sci., 4(9): 721-727.
20. Feng, J., Hu, Y., Ma, Z., Yu, Q., Zhao, J., Liu, X. and Zhang, G. (2012) Virulent avian infectious bronchitis virus, people’s republic of China. Emerg. Infect. Dis., 18(12): 1994-2001.
21. Amit, G., Hanish, D., Pal, J.K. and Prajapati, K.S. (2010) Isolation, identification and molecular characterization of IBV variant from outbreak of visceral gout in commercial broilers. Vet. World, 3(8): 375.
22. Sid, H., Fettah, A. and Lounas, A. (2011) Descriptive study of an outbreak of avian urolithiasis in a large commercial egg complex in Algeria. Not. Sci. Biol., 3(1): 22-25.
23. De Wit, J.J. (2000) Detection of infectious bronchitis virus. Avian Pathol., 29(2): 71-93.
24. Lashgari, M.S. and Newman, J.A. (1984) Serological comparison and antigenic relationships of seven serotypes of infectious bronchitis virus using the haemagglutination-inhibition test. Avian Dis., 28(2): 435-443.
25. Brown, A.J. and Bracewell, C.D. (1985) Application of the haemagglutination inhibition test to typing of infectious bronchitis virus. Vet. Rec., 116(2): 47-48.
26. Cook, J.K.A., Brown, A.J. and Bracewell, C.D. (1987) Comparison of the haemagglutination inhibition test and the serum neutralization test in tracheal organ cultures for typing infectious bronchitis virus strains. Avian Pathol., 16(3): 505-511.
27. Eterradossi N, Britton P: Avian infectious bronchitis, in: Biological Standards Commission (Ed.), Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organisation for Animal Health, Paris, France. http://www.oie.int/fileadmin/Home/eng/Health_standards/ tahm/2.03.02_AIB.pdf. Last accessed on 15-11-2018.
28. De Wit, S., Cook, J.K.A. and Van Der Heijden, H.M.J. (2011) Infectious bronchitis virus variants history, current situation and control measures. Avian Pathol., 40(3): 223-235.
29. Liu, S.W., Zhang, Q.X., Chen, J.D., Han, Z.X., Liu, X., Feng, L., Shao, Y.H., Rong, J.G., Kong, X.G. and Tong, G.Z. (2006) Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Arch. Virol., 151(6): 1133-1148.
30. Kannan, G., Ball, C. and Forrester, E. (2015) Genotypes of infectious bronchitis viruses circulating in the Middle East between 2009 and 2014. Virus. Res., 210: 198-204.
31. Kusters, J.G., Niesters, H.G.M., Lenstra, J.A., Horzinek,M.C. and Van Der Zeijst, B.A.M. (1989) Phylogeny of antigenic variants of avian coronavirus IBV. Virol. J., 169(1): 217-221.
32. Alexander, D.J., Allan, W.H., Biggs, P.M., Bracewell, C.D., Darbyshire, J.H., Dawson, P.S., Harris, A.H., Jordan, F.T., Macpherson, I., Mcferran, J.B., Randall, C.J., Stuart, J.C., Swarbrick, O. and Wilding, P. (1983) A standard technique for haemagglutination inhibition tests for antibodies to avian infectious bronchitis virus. Vet. Rec., 113(3): 64.
33. Cook, J.K., Chesher, J., Baxendale, W., Greenwood, N., Huggins, M.B. and Orbell, S.J. (2001) Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathol., 30(4): 423-426.
34. Jackwood, M.W. (2012) Review of infectious bronchitis virus around the world. Avian Dis., 56(4): 634-641.
35. Terregino, C., Toffan, A., Beato, M.S., De Nardi, R., Vascellari, M., Meini, A., Ortali, G., Mancin, M. and Capua, I. (2008) Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen-free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol., 37(5): 487-493.
36. Sasipreeyajan, J., Pohuang, T. and Sirikobkul, N. (2012) Efficacy of different vaccination programs against Thai QX-like infectious bronchitis virus. Thai. J. Vet. Med., 42(1): 73-79.
So nice but still how to control the present outbreak? Can you give us further guidelines? Your study is excellent.










.jpg&w=3840&q=75)