INTRODUCTION
Virginiamycin (Vm) is a cyclic polypeptide antibiotic complex from Streptomyces virginiae of the streptogramin class of antibiotics. It is commonly used to treat infections from gram-positive organisms or as a growth promotant in cattle, swine, and poultry diets. The Vm complex consists of 2 major components, Vm factor m1 and s1, which act synergistically to prevent protein synthesis within bacteria (Cocito, 1979). It also has been suggested that antibacterial agents improve growth performance and nutrient utilization by either thinning the epithelium of the small intestine or by reducing the production of growth depressing toxins or metabolites by intestinal microflora (Cocito, 1979).
Several researchers have reported the nutrient-sparing effects of Vm on CP, energy, Ca, Mn, and P in pigs and chickens. Pelura et al. (1980) reported that the addition of Vm to low CP diets improved BW gain and feed efficiency in pigs. Agudelo et al. (2003) reported that Vm improved P, Ca, and Zn digestibility and retention in pigs when compared with those fed the control diet. Cervantes et al. (2002) reported that the addition of Vm to marginally deficient P diets for broilers increased BW, improved feed conversion, and decreased mortality. These recent results agree with the findings of Buresh et al. (1985), who reported that the addition of Vm to diets with 0.47% total P or greater increased BW and P utilization
A significant proportion of the endogenous Ca and P in corn and soybean meal (C-SBM) is bound to phytate (Harland and Oberleas, 1999; Jang et al., 2003). The phytate-bound minerals have a reduced bioavailability to nonruminant animals, resulting in an increased need for inorganic P supplementation (Broz et al., 1994; Johnston and Southern, 2000). Moore et al. (1999) indicated that the biggest problem facing the poultry industry is P runoff into surrounding watersheds. If Vm can increase P utilization as indicated by Buresh et al. (1985) and Agudelo et al. (2003), then Vm could possibly reduce P runoff by increasing P bioavailability to the animal. Therefore, the objectives of these experiments (EXP) were to investigate the effects of Vm in diets with adequate or reduced levels of Ca and nonphytate P (nPP) on growth performance and bone response variables in broilers.
MATERIALS AND METHODS
General
The methods used in these EXP were approved by the Louisiana State University Agricultural Center Animal Care and Use Committee. Each EXP used female Ross × Ross broilers obtained from a commercial hatchery on d 0 post-hatching. The broilers were allotted, wing-banded, and placed in environmentally controlled brooder batteries (Petersime Incubator company, Gettysburg, OH) with raised wire floors for the duration of the 0- to 18-d EXP.
The diets used for these EXP were C-SBM based and were formulated to provide 1.26% total Lys and 3,200 kcal of ME/kg of diet (Table 1). The diets met or exceeded the nutrient requirements suggested by the NRC (1994) except for Ca and nPP when appropriate. The reduced Ca and nPP levels were achieved by replacing limestone and monocalcium phosphate with sand.
Throughout all EXP, the chicks, feed, and water were checked twice daily, and the feed in mash form and water were provided ad libitum. At the termination of each EXP, all broilers and feed were weighed for calculation of daily gain (ADG), daily feed intake (ADFI), gain:feed (G:F), Ca intake, and nPP intake. Calcium intake was calculated by multiplying the Ca content of the diet by ADFI. Nonphytate phosphorus intake was calculated in the same manner. After weighing, 3 (EXP 1 and 3) or 6 (EXP 2 and 4) broilers per replicate were randomly selected for bone analyses. The left tibia was collected, pooled by replicate, and then frozen until further analyses.
EXP 1 and 2 In EXP 1
and 2, 216 broilers per EXP were allotted to 6 treatments with 6 replications of 6 chicks each. The average initial and final BW for EXP 1 and 2 were 47 and 488, and 40 and 523 g, respectively. The treatments were as follows: Diet 1) C-SBM with 1.00% Ca and 0.45% nPP (positive control; PC); Diet 2) C-SBM with 0.80% Ca and 0.45% nPP (0.80Ca); Diet 3) C-SBM with 1.00% Ca and 0.35% nPP (0.35nPP); and Diets 4 to 6) as Diets 1 to 3 supplemented with 11 (EXP 1) or 22 (EXP 2) ppm of Vm (Stafac 20, Phibro, Fairfield, NJ).
EXP 3
In EXP 3, 240 broilers were allotted to 6 treatments with 8 replications of 5 chicks each. The average initial and final BW were 45 and 462 g, respectively. The treatments were as follows: Diet 1) C-SBM with 1.0% Ca and 0.45% nPP (PC); Diet 2) C-SBM with 0.70% Ca and 0.45% nPP (0.70Ca); Diet 3) C-SBM with 1.00% Ca and 0.25% nPP (0.25nPP); and Diets 4 to 6) as Diets 1 to 3 supplemented with 9 ppm of Vm.
EXP 4
In EXP 4, 432 broilers were allotted to 12 treatments with 6 replications of 6 chicks each. The average initial and final BW were 37 and 432 g, respectively. Four levels of nPP (0.15, 0.25, 0.35, or 0.45%) and 3 levels of Vm (0, 11, or 22) were used in a 4 × 3 factorial arrangement.
Bone Analysis
After thawing the bones, bone breaking strength (BBS) was determined (HD 250 Texture Machine, Texture Technologies Corporation, Scarsdale, NY) using a 3-point bend rig with a load cell capacity of 25 kg and cross-head speed of 100 mm/min. After determining BBS, fat was extracted from the tibias by a 36-h Soxhlet extraction in ethyl alcohol followed by a 36-h extraction with diethyl ether. The bones were dried at 110°C for 24 h and then weighed. The dry, defatted tibias were dry-ashed in a muffle furnace at 560°C for 24 h. Bone ash percentage (BAP) was not determined in EXP 3. After cooling, tibia ash was weighed for determination of ash weight, BAP, BBS per Ca intake (BBS/Ca), BBS per nPP intake (BBS/nPP), milligrams of tibia ash per chick (ASH), tibia ash per Ca intake (ASH/ Ca), and tibia ash per nPP intake (ASH/nPP) for EXP 1, 2, and 4. Bone breaking strength per Ca or nPP intake was calculated by dividing the kilograms of BBS by total Ca or nPP intake in grams. Tibia ash was calculated for each treatment by dividing the ash weight of the tibias by the number of tibias. Tibia ash was then divided by total Ca or nPP intake to calculate ASH/Ca and ASH/ nPP, respectively.
Statistical Analysis
Data were analyzed by ANOVA procedures (Steel and Torrie, 1980) for completely randomized designs by the GLM procedure of SAS (SAS Inst., Inc., Cary, NC). In all EXP, contrast statements were used to test the main treatment effects and any interactions of Ca, nPP, and Vm. The pen of chicks served as the experimental unit for all response variables. Treatment differences were considered significant at α ≤ 0.10.
RESULTS
EXP 1
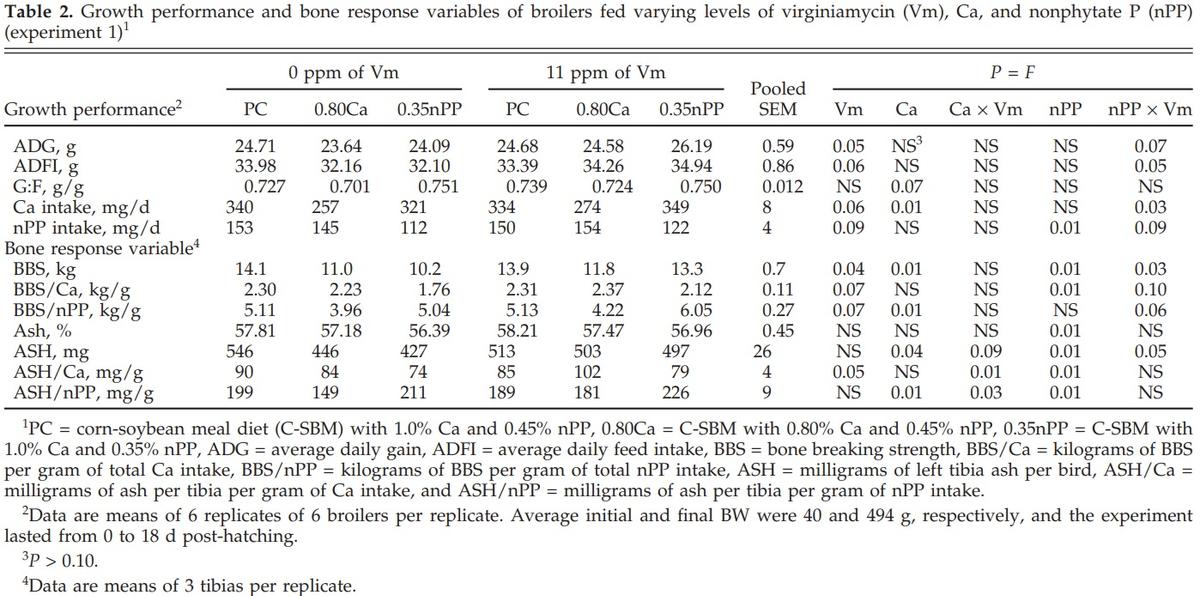
Reducing dietary Ca decreased (P < 0.01 to 0.07) G:F, Ca intake, BBS, BBS/nPP, ASH, and ASH/nPP (Table 2). Reducing dietary nPP decreased (P < 0.01) nPP intake, BBS, ASH, BAP, BBS/Ca, and ASH/Ca, but it increased (P < 0.01) ASH/nPP. The addition of Vm increased (P < 0.04 to 0.09) ADG, ADFI, Ca intake, nPP intake, BBS, BBS/Ca, BBS/nPP, and ASH/Ca. The addition of Vm to the 0.80Ca diet increased (Ca × Vm interaction, P < 0.01 to 0.09) ASH, ASH/nPP, and ASH/Ca. The addition of Vm to the 0.35nPP diets increased (nPP × Vm interaction, P < 0.03 to 0.10) ADG, ADFI, Ca intake, nPP intake, BBS, BBS/Ca, BBS/nPP, and ASH.
EXP 2
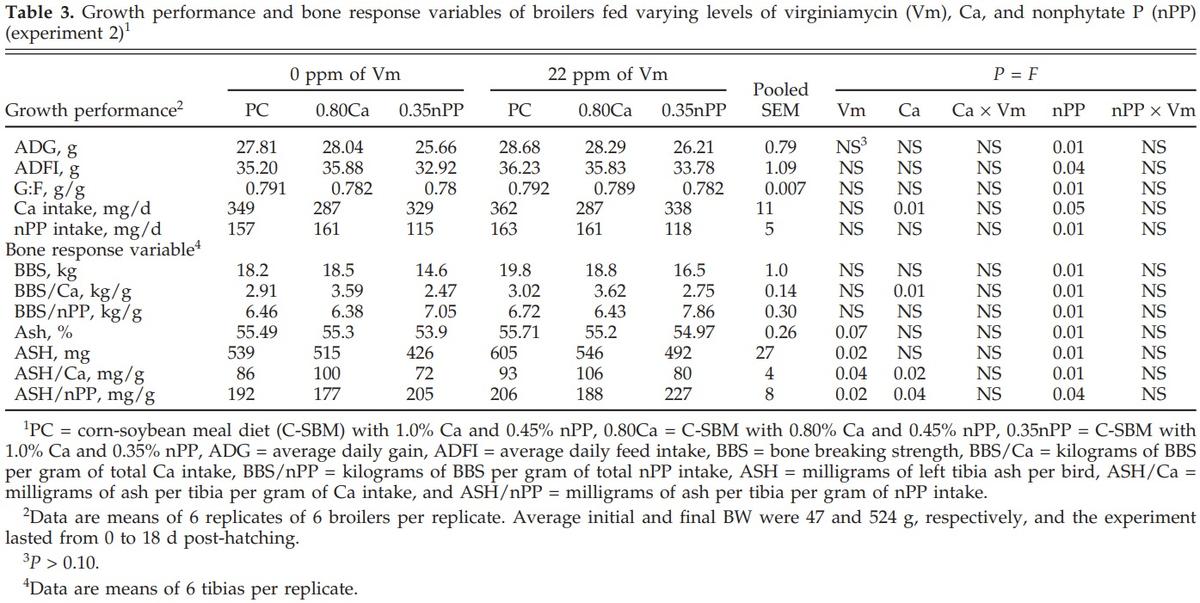
Reducing dietary Ca decreased (P < 0.01 to 0.04) Ca intake and ASH/nPP, but it increased (P < 0.02) BBS/Ca and ASH/Ca (Table 3). Reducing dietary nPP decreased (P < 0.01 to 0.05) ADG, ADFI, G:F, Ca intake, nPP intake, BBS, BBS/Ca, BAP, ASH, and ASH/Ca, but it increased (P < 0.01 to 0.04) BBS/nPP and ASH/nPP. The addition of Vm increased (P < 0.02 to 0.07) BAP, ASH, ASH/Ca, and ASH/nPP, but there were no interactive effects of Vm and Ca or nPP. The lack of an interaction was because Vm had equally positive effects in the PC diet and in the reduced Ca and nPP diets.
EXP 3
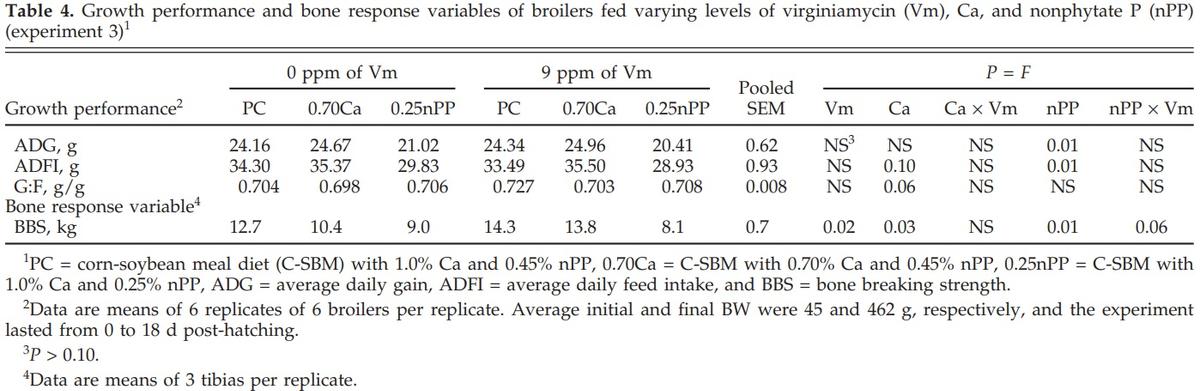
Reducing dietary Ca decreased (P < 0.03 to 0.06) G:F and BBS, while it increased (P < 0.10) ADFI (Table 4). Reducing dietary nPP decreased (P < 0.01) ADG, ADFI, and BBS. The addition of Vm increased (P < 0.02) BBS in birds fed the PC diet but decreased (nPP × Vm, P < 0.06) BBS in birds fed the 0.25nPP diet.
EXP 4
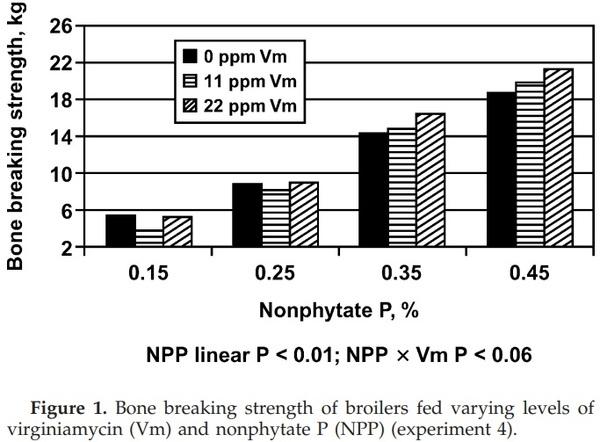
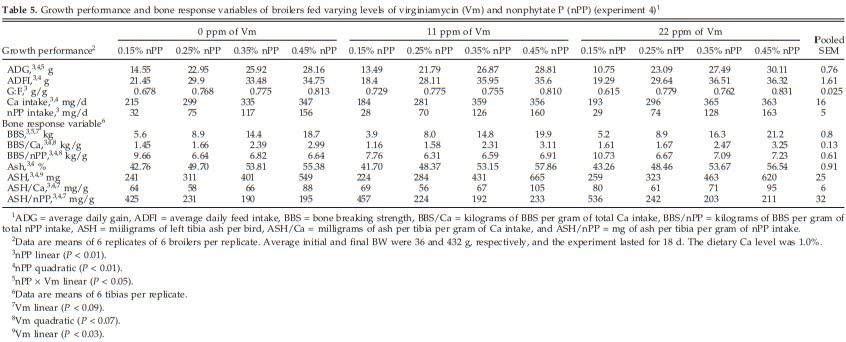
Increasing dietary nPP increased (nPP linear and/or quadratic, P < 0.01) ADG, ADFI, G:F, Ca and nPP intake, BBS, BBS/Ca, BAP, ASH, and ASH/Ca (Table 5). Increasing dietary nPP decreased (P < 0.01) BBS/nPP and ASH/ nPP. The addition of Vm increased (Vm linear, P < 0.03 to 0.09) BBS, ASH, ASH/Ca, and ASH/nPP. The addition of 11 ppm of Vm decreased (Vm quadratic, P < 0.07) BBS/ Ca and BBS/nPP, but the 22-ppm addition increased these response variables (Vm quadratic, P < 0.07). The addition of Vm increased (nPP × Vm, P < 0.05) ADG and BBS in broilers fed diets containing 0.35 and 0.45% nPP but not in diets containing 0.15 or 0.25% nPP (Figure 1).
DISCUSSION
Increasing dietary Ca from 0.7 or 0.8% to 1.0% increased G:F and many bone response variables, but it did not affect ADG or ADFI. The lack of increase in ADG and ADFI as Ca levels were increased agrees with the findings of Applegate et al. (2003), who reported that increasing the level of Ca supplementation from 0.4 to 0.9% had no effect or decreased ADG from 7 to 21 d or 14 to 24 d of age, respectively, but these data disagree with findings of other researchers who reported that ADG, ADFI, and G:F were increased as dietary Ca levels were increased (Boling-Frankenbach et al., 2001; Onyango et al., 2003).
Increasing dietary nPP increased all growth variables and many bone response variables. These results agree with the findings of other researchers. Onyango et al. (2003) reported that growth performance variables and tibia ash were increased linearly, and tibia bone mineral content and density were increased linearly and quadratically as dietary concentrations of P were increased.
The purpose of this research was to determine whether Vm increased the availability of Ca and nPP beyond its effect as a growth promotant. In EXP 1, reducing nPP to 0.35% decreased BBS and ASH, and the addition of Vm increased these response variables. However, Vm also increased feed intake; thus, the increase in BBS and ASH could be due to an increase in nPP intake and not to increased nPP utilization. However, BBS/nPP was also increased, suggesting that Vm increases nPP utilization. The addition of Vm also increased ASH/Ca, but the effect was not significant. In EXP 2, reducing nPP to 0.35% decreased growth and all bone response variables, and the addition of Vm improved or tended to improve many of these response variables. However, Vm was just as effective in the PC diet as the diet deficient in nPP. In EXP 3, reducing nPP levels to 0.25% decreased ADG, ADFI, and BBS. The addition of Vm did not improve any of these response variables, and in fact, Vm decreased BBS in birds fed 0.25% nPP. In EXP 4, decreasing dietary nPP levels decreased ADG and BBS, and there were significant Vm × nPP interactions. Virginiamycin decreased or did not affect ADG or BBS in birds fed 0.15 or 0.25% nPP, respectively, but Vm increased ADG and BBS in birds fed 0.35 or 0.45% nPP.
The results of this research suggest that Vm may not affect Ca utilization beyond its effects as a growth promotant. In EXP 1 (0.80Ca) and EXP 3 (0.70Ca), BBS was decreased in the low Ca diets. In EXP 1, the addition of Vm did not affect BBS in chicks fed the low Ca diet, and in EXP 3, BBS was increased by the addition of Vm in birds fed the low Ca diet and the PC diet.
Buresh et al. (1985) reported that Vm decreased the amount of P needed to increase BW. Agudelo et al. (2003) reported a 28% increase in P digestibility, an 11% increase in Ca digestibility, and an increase in absolute retention of P, Ca, Zn, and Mg in limit-fed pigs given Vm. Henry et al. (1986) reported that the addition of Vm increased Mn utilization regardless of the level of Mn supplementation and reduced intestinal weight in broilers. The reduction of intestinal weight was attributed to a thinning of the intestinal wall.
There are several possible reasons for the nutrient-sparing effects of Vm. One possibility is that Vm acts on the microflora's biochemical processes in the cell (Parks et al., 2001). Virginiamycin inhibits bacterial protein synthesis by binding to the 50s ribosomal subunit and blocks normal peptide formation (Cocito et al., 1974). This inhibition of protein synthesis gives Vm a broad-spectrum action on bacteria such as Lactobacillus, Bifidobacterium, and Streptococcus, which may be responsible for retarded growth in broilers (Cummings, 2003). Thus, by eliminating these harmful small intestine microflora, Vm may cause more nutrients to be available for the animal and not diverted to microflora.
The failure of Vm to increase growth and bone response variables at dietary nPP levels < 0.35% was not an anticipated response. One possible explanation for the lack of response to Vm at nPP levels of ≤0.25% could be an imbalance of Ca:nPP. Hulan et al. (1985) reported that as Ca:nPP was increased from 2.53:1 to 3.38:1 in broiler diets, BW was decreased. Larger Ca:total P have been reported to decrease the effectiveness of phytase in broilers at nPP levels of 0.27% (Qian et al., 1997). The increase in Ca:nPP in EXP 3 and 4 from 2.22:1 in the PC diet to 6.67:1 in 0.15% nPP could have similar effects on the effectiveness of Vm. Buresh et al. (1985) reported that Vm did not affect BW below 0.47% P, but the addition of Vm to diets > 0.47% P resulted in an increase in BW, feed intake, and P utilization, which is consistent with our findings.
In summary, Vm seems to improve nPP utilization, but it is difficult to separate this effect from the effect of Vm as a growth promotant. In one EXP but not another, Vm seemed to improve nPP utilization when dietary nPP level was 0.35%. Virginiamycin had no or negative effects when the level of nPP was ≤0.25%. In another EXP, Vm improved many of the response variables in birds fed the 0.35% nPP diet, but it was equally effective at 0.45% nPP, which is at or above the requirement. The implications of this increased utilization are numerous, such as a potential reduction in the amount of Ca and nPP that needs to be supplemented to C-SBM diets and a reduced loss of these nutrients to the environment. Further research is needed to ascertain the mode of action of Vm and its potential to increase the utilization of Ca and nPP in CSBM diets and to investigate the relationship between Ca:nPP and Vm.
This article was originally published in 2005 Poultry Science 84:1868–1874. https://doi.org/10.1093/ps/84.12.1868. This is an Open Access article under a Creative Commons Attribution License. 









.jpg&w=3840&q=75)