The Effect of Early Feeding and Feed Additives on Lymphoid Organs, Intestinal Microbiology and Meat Peroxidation of Broiler
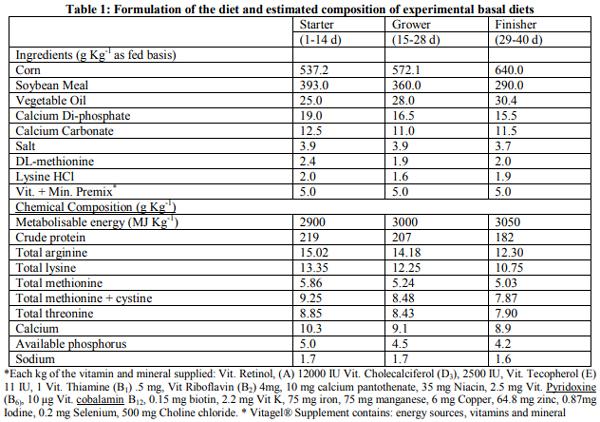
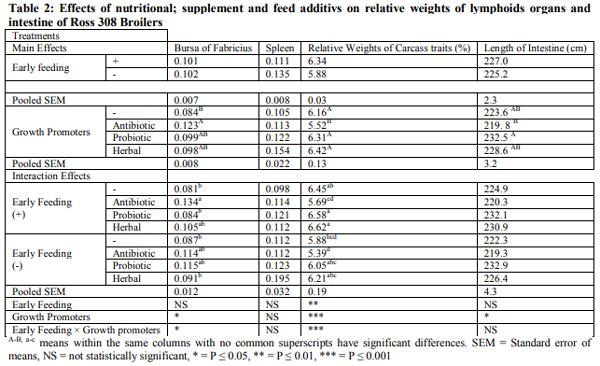
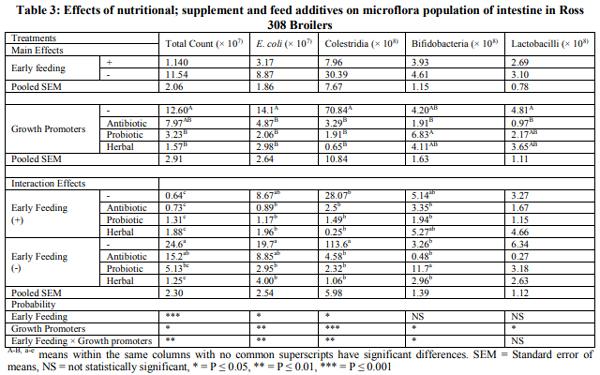
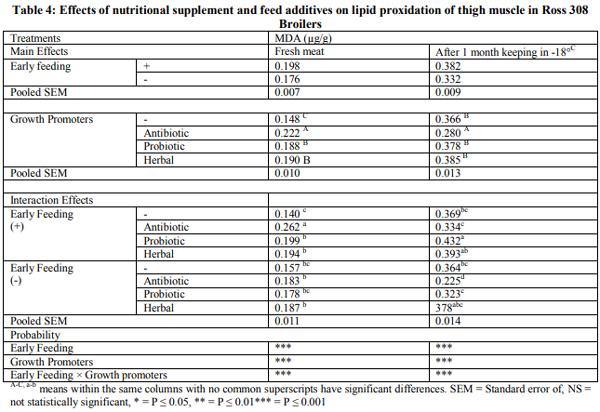
Early feeding of newly hatched chicks may improve immunity system and intestinal development. This trial was carried out to evaluate the effect of immediate access to nutritional supplement post hatch and feed additives on lymphoid organs, meat stability and gut microbiology of broiler chicks under commercial condition. A total of 1200 male chicks were allotted according to two group in hatchery: Fed groups received early feeding (Vitagel®) during transport until 24 hours post hatch and deprived groups were restricted within transport box for 24 hours post hatch without Vitagel®. In farm, the birds divided into negative control (basal diet) and positive control (Availmycin as antibiotic; Bacillus subtillis as probiotic, and Bioherbal® as medicinal plants. When broiler chicks were fed immediately post hatch decreased detrimental bacteria (Clostrodium Spp and E.Coli) (P<0.05).The friendly bacteria (Lactobacilli and Bifidobacteria) were not changed (P<0.05). Feed additives showed significantly reduced (p<0.001) detrimental bacteria and increased friendly bacteria (p<0.05). The interactive effect between early feeding supplement and feed additives affected intestinal microbiology (P<0.01) through increasing friendly bacteria and reducing detrimental bacteria. At market age, Immediate access to feed increased meat peroxidation (MAD) of broiler chicks in fresh samples (at one day of chilling -18ºC) and stored sample (at 30day of chilling -18ºC (P<0.05), while feed additives had the similar effect. Interactive effects was observed for meat stability (P<0.05). In conclusion, early feeding by Vitagel® may be suitable for broiler chicks that exposure to nutritional and hatchery stresses.
Key word: Initial period, nutritional supplement, Broiler meat Stability.
1. Ahmed A.M. A comparative study on the effect of some growth promoters in chicken. Ph.D. Thesis. Veterinary Medicine Science. Pharmacology. Zagazig University. Egypt, 2007.122.
2. AL-Ankari AS, Zaki, MM, Alsultan, SI. Use of habakmint (menthalongifolia) in broiler chicken diet. Inter J Poult Sci 2004; 3:629-634.
3. Alharth, M. A Efficacy of vegetable diet with antibiotic s and different types of spices or their mixtures on performance, economic efficiency and carcass traits of broiler. J Agri Sci 2001; 29:901-913.
4. Aluwong T, Kawn M, Rajy M, Dzenda J, Govwany F, Sinkalu V.J, Ayo J. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chicks. Antioxidant, 2013; 2:326-339.
5. Awaad, M. H. H., Elmenawey M, Ahmed K. A. Effect of specific combination of Carvacrol, Cinnamaldehyde and Capsicumo Leorsin on the growth performance, Carcass quality and gut integrity of broiler chicken. Veterinary World 2014, 7 (3):284-290.
6. Bhanja S, K., Anjali Devi C, Panda A. K., Shyam Sunder G. Effect of post-hatch nutrient intubation on performance, intestinal growth, meat yield and immune response in broiler chickens. Asian Australas. J. Anim. Sci., 23:515- 520.
7. Bidarnamani E, Shargh M.S, Dastar B, Daran S.Z. Effect of different levels of Agaricus Bisporus mushroomwast with or without probiotic on meat quality of broiler chicken. Advance Environmental biology 2015; 9(5):421- 427.
8. Branton S, Lott B, Morgan GW, Deaton, JW. Position of meckel's diverticulum in broiler type chicken. Poult Sci 1988; 67:677-679.
9. Burt, S). Essential oils: their antibacterial properties and potential applications in foods a review. Inter J Food Microb 2004; 94, 223-253.
10. Cengiz O, Koksal B.H, Tatli O, Sevium O, Avei H, Epikmen T, Beyaz D, Buyukyoruk S, Boyacioglu M, Uner A, Onol A. Influence of dietary organic acid blend supplementation and interaction with delayed feed access after hatch on broiler growth performances and intestinal health. Veterinarni Medicina 2012, 56(10):515- 528.
11. Cheema, M.A, Qureshi, M.A, Havenstein G.B, Ferket P.R, Nestor K.E. A comparison of the immune response of 2003 commercial turkeys and a 1966 random bred strain when fed representative 2003 and 1966 turkey diets. Poult Sci 2007;86:241- 248.
12. Coetzee GY.M., Hoffman,L.C. Effect of dietary vitamin E on the performance of broilers and quality of broiler meat during refrigerated and frozen storage. South Afr j Anim Sci, 2001;31(3):161-175.
13. Correa, AP.F, Daroit, D.J, Coelho J.G, Meira S.M.M, Loopes, F.C.; Segalin J.; Risso P.H., Brandelli, A. Antioxidant of ovin milk caseinate hydrolyzed with a microbial protease J Sci Food Agric, 2011;91(21):2247-54.
14. Dankowiakoska A.; Kozlowska I., Bendnarczyk M. Probiotic, prebiotic and symbiotic in poultry. Mode of action, limitation and achievements. Journal of Central European Agriculture, 2013; 14 (1): 467-478.
15. Daskiran M; Oonol A.G; Cengiz O, Unsal, H, Turky lmaz S, Tatli, O, Sevim, O. Influence of dietary probiotic inclusion on growth performance, blood parameters and intestinal microflora of male broiler chickens exposed to post hatch holding time. J Appl Poultry Res, 2012; 21:612-622.
16. Diarra S.S, Sanda Kabatu, D, Perera, D, Tabuaciri, P, Mohammed U. Growth performance, carcass measurements and organs weight of broiler chickens fed Cassava copra based on commercial finisher diets in somoa. Asian Australas. J. Anim. Sci, 2014; 8(1):16-22.
17. Dibner, J. J, Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poultry Res, 2002; 11(4): 453–463.
18. Dibner J. J, Knight C. D, Kitchell, M. L, Atwell C. A, Downs, A. C, Ivey F. J. Early feeding and development of the immune system in neonatal poultry. J. Appl. Poultry Res, 1998;7(4),425-436.
19. Durrani FR, Ismail M, Sultan A, Suhail SM, Chand N, Durrani Z. Effect of different levels of feed added turmeric (Curcuma longa) on the performance of broiler chicks. Journal of Agricultural and Biological Science 2006;1,9-11.
20. El-sayed, A.S.S. Some nutrition studies on feed additives in broiler chickens. Master Thesis. Veterinary Medicine College. Zagazig University. Egypt, 2007, 112.
21. Engber G, Ranjitkar, S.T., Louridsen C. The influence of early nutrition on body weight, gastrointestinal microflora and humeral immunology of broiler. 19th European Symposium on Poultry Nutrition. Postdam/Germany. August 26-24,2013.
22. Fahimeh Alipour; Ahmed Hassanbadi; Abolghasem Golian and Hassan Nassiri Moghaddam. Effect of plant extracts derived from thyme on male broiler performance. Poult Sci, 2015;94:2930-2634.
23. Fridman A, Bar-Shira, E. Effect of nutrition on development of immune competence in chicken's gut–associated lymphoid system. Proceeding of the 15th European Symposium on Poulty Nutrition, Balatonfured, Hungary, 25-29, September, 2005. Pp 247-255. World's Poultry Science Association (WPSA).
24. Gonzales E, Kondo N, Saldanha, E.S.P.B, Loddy, M.M, Careghi C, Decuypere, E Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poul Sci, 2003;82:1250-1256.
25. Gudev D, Popova-Ralcheva S, Moneval P, Bonovska M, Valchev G, Valcheva, A. Effect of supplemental sangrovit on some biochemical indices and leukocytes phagocytic activity in growing pigs. Archivos de Zootecnia, 2004; 7:123-34.
26. Guo F., Kwakkel R, Williams, B., Parmentier, H., Li, W., Yang, Z, Verstegen, M., Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of eimeria tenella-infected chickens. Poult Sci, 2004, 83, 1124-1132.
27. Hangalapura B.N, Nieuwland, M.G.B, Devries Reillingh G, Buyse J, Vandde Brabdt H, Kemp, B. and Parmentier, H.K. Severe feed restriction enhances innate immunity but suppresses cellular immunity in chicken lines divergently selected for antibody responses. Poult Sci, 2005; 84:1520- 1529.
28. Hossain, M.M; Begum, M.; Kim, I.M. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospore on growth performance, nutrient digestibity, meat quality, relative organ weight, microbial shedding and excretanoxious gas emission in broiler. Veterarni Medicina, 2015;60 (2): 77-86.
29. Jang, A. Liu, X.D.; Shin, M.H.; Lee, B.D.; Lee, J.H. and Jo. c. Antioxidative potential of raw breast meat from broiler chicks fed adietary medicinal herbal extract mix. Poultry Science, 2008. 87:2382-2389.
30. Jung S, Choe JH, Kim B, Yun H, Kruk Z, JOCH. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Science, 2010; 86:612-618.
31. Koksal, B.H, Cengiz O., Sevim O, Tatli O, Uner A.G, Beyaz, D, Buyukyoruk S, Onol, A.G. Use of prebiotic supplementation to diet for reducing the negative effect of delayed feed access on growthrate in broiler chicken. Inter j poult sci. 2013; 12(12): 714 -720.
32. Kuserv J, Jantosovic, J, Saly J, Kozak, M. The effect of vitamin E on the quality of fat component of broiler chicken meat. Veteriarni Medicina, 1996;41(5):139-142.
33. Lesson, S. and Summers, D. Nutrition of the chicken. (4edth). Published by University books. Guelph, Ontario, Canada. 2001; Pp440-443.
34. Maiorka, Dahlke. Broiler adaptation to post – hatching period. Ciencia Rural, Santa Maria 2006, 36(2):701-708.
35. Marchini, C.F.P, Silva AP. L, Nascimento M.R, Beletti M.E., Silva N.M., Guimarraes, E. C. Body weight, intestinal morphometry and cell proliferation of Broiler chicken submitted to cyclicheat stress. Int J poult Sci, 2011;10(60):455 -460.
36. Marcincak, S, Popelka P., Bystricky P, Hussein K, Tudecova K. Oxidative stability of meat products after feed of broiler chickens with additional amounts of vitamin E and Rosemory. Sijecanj –Veljacarbr, 2005;VII(1):34-39.
37. Mathivanan R., Kalaiarasi, K. Panchagavya and Andrographis Panculata as alternatives to antibiotic growth promoters on hematological, Serum Biochemical parameters and immune status of broilers. Poult Sci. 2007;44:198-204.
38. Mead, G. C, Adams, B. W. Some observations on the caecal microflora of the chick during the first two weeks of life. Brit Poult Sci, 1975; 16(2): 169–176.
39. Mehala C and Moorthy M. Production performance of broilers fed with Aloe vera and Curcuma longa (turmeric). Int J Poult Sci, 2008; 7, 852-856.
40. Mountnery GJ. Reduced sodium usage in poultry muscle foods. Food Technol, 1976; 7:60.
41. Nickos A botsoglou, Dimitrios J Fletouris, Georgios E. Papageorgiou, Vassilios N. Vassilopoulos, Antonios J. Mantis AND antonios G. Trakatellis. Rappid Sensitive and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food and feed stuff samples. J. Agric. Food Chem., 1994;42(9):1931-1937.
42. Nnadi, P.A. Eze, PC, Ezema WS. Influence of delayed feeding on the performance, Development and response of immune system to Newcastle disease vaccination in chicken. Int J Poult Scie, 2010; 9 (7):669-674.
43. NoyY, Sklan, D. Energy utilization in newly hatched chicks. Poult Sci, 1999;78(12), 1750- 1756. 44. NRC. National Research Council. Nutrient requirements of Poultry. 9th revised. ed. National Academy Science., Washington. DC. 1994.
45. Ocak N, Erener, G. Performance of broilers fed diets supplemented with dry peppermint (Menthapiperita L) or thyme (Thymus vulgaris L.) leaves as growth promoter source. Czech Journal of Animal Science, 2008;53: 169-175.
46. Pall L, Dublecz, K, Husveth F, Kulcsar, M. Early nutrition affect plasma corticosterone of broiler chicks. 19th European symposium on poultry nutrition. August 26-29, Potsdam-Germany 2013.
47. Pan M., Jiang, J.S., Pan, J.L. Antioxidant activities of rapeseed protein hydrolysate. Food and Bioprocess Technology 2011, 4:1144-1152.
48. Parksang Oh, Ryu Chaemin, Park Byung-Sang, Hwangho Jong. The meat quality and growth performance in broiler chicken, fed diet with Cinnamon powder. Journal of Environmental biology 2013,34:127-133.
49. Petracci M, Cavani C. Muscle growth and poultry meat quality issues: Review. Nutrients 2012,4 (1):1-12.
50. Pires DL; Malheiros, EB.; Boleli, JC. Influence of sex, age of fasting on blood parameters and body bursa, spleen, yolksac weight of broiler chicks. Brazilian Journal of Poulty Sci, 2007;9:221-228.
51. Potturi P.V, Patterson J.A., Applegate T.J. Effects of delayed placement on intestinal characteristics in turkey poults. Poult Sci 2005,84:816-824.
52. Rahimi S, Teymouri Zadeh Z, Karimi Torshizi M.A, Omidbaigi R., Rokni H. E ffect of three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chicken. Journal of Agricultural Science and Technology 2011,13:527-539.
53. Sarker MSK, Park S.R, Kim, G.M, Yang, C.Y. Hamcho (Salicornia herbacea) with probiotic as alternative to antibiotic for broiler production. Journal of Medicinal Plant Research 2010; 4(5):415-420.
54. Simone Pophal, Paul, E. Modziak, Serogiol Vieira. Statellite cell mitotic activity of broiler feed differing levels of lysine. Int J Poul Sci, 2004;3(12):758-763.
55. Sung S.H, Bae, Y. S, Oh, S.H, Lee I, Kim H.J, Jo C. Possibility of instrumental differentiation of duck breast meat with different processing and storage condition. Korean J Food SCI An Resources 2013,33(1);96-102.
56. Uni Z, Ferket, RP. Methods for early nutrition and their potential. Worlds Poult Sci J 2004;60:101-111.
57. Van Dono, ND. Nutrition strategies to improve enteric health and growth performance of poultry in the post antibiotic era. Ph.D. Thesis. Veterinary Medicine College, Glasgow, University. 2012, 22.
58. Yadav, G.B, Kadam, A.S, Pachpande, A.M.; Lambate, V.D, Maini, S., Ravikanth, K. Post hatch histo –morphological studies of small intestinal development in chicks fed with herbal chick nutritional supplement. Int J poult Sci, 2010;9 (9):851-855.
59. Zahid B, Saleen, G, Aslam, A.; Imran, MI, Young M. O Effect of immunostimulants on humoral response against bursal disease in broiler. PAK VET J 2015;35(2):227-230.
60. Zhang H.M, Hunt, H.D., Kulkarni, G.B., Palmquist, D.E, Bacon L.D. Lymphoid organ size varies among inbred lines 63and 72 and their thirteen recombinant congenic strains of chickens with the same major histocompatability complex. Poult Sci, 2006;85:844-85.







.jpg&w=3840&q=75)