Simultaneous Carriage of mcr-1 and Other Antimicrobial Resistance Determinants in Escherichia coli From Poultry
Author details:
1 Laboratorio de Bacteriología General, Instituto de Patobiología, Centro Nacional de Investigaciones Agropecuarias, Instituto Nacional de Tecnología Agropecuaria, Buenos Aires, Argentina; 2 Consejo Nacional de Investigaciones Científicas y Tecnológicas, Buenos Aires, Argentina; 3 Laboratorio de Resistencia Bacteriana, Cátedra de Microbiología, Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Buenos Aires, Argentina.
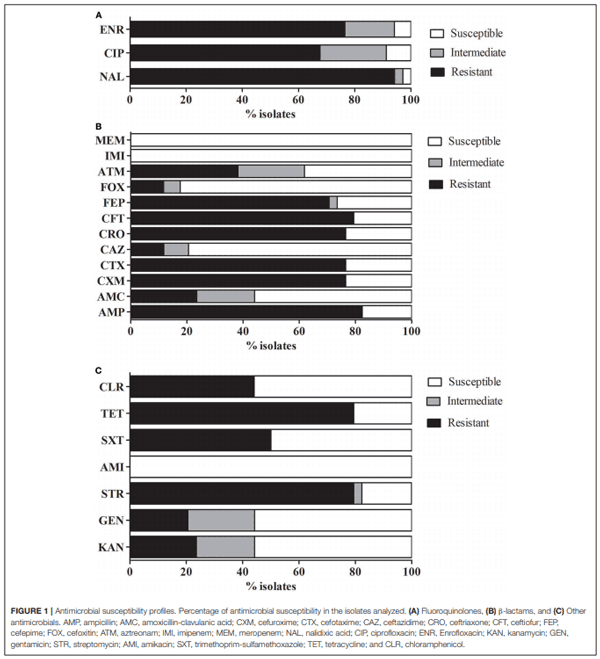
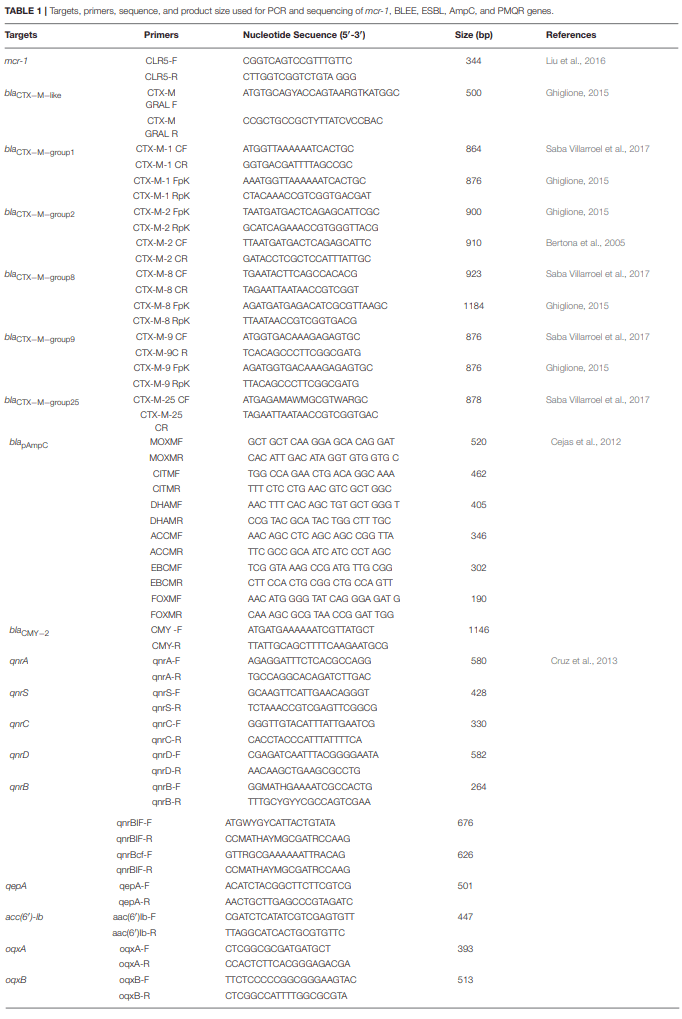
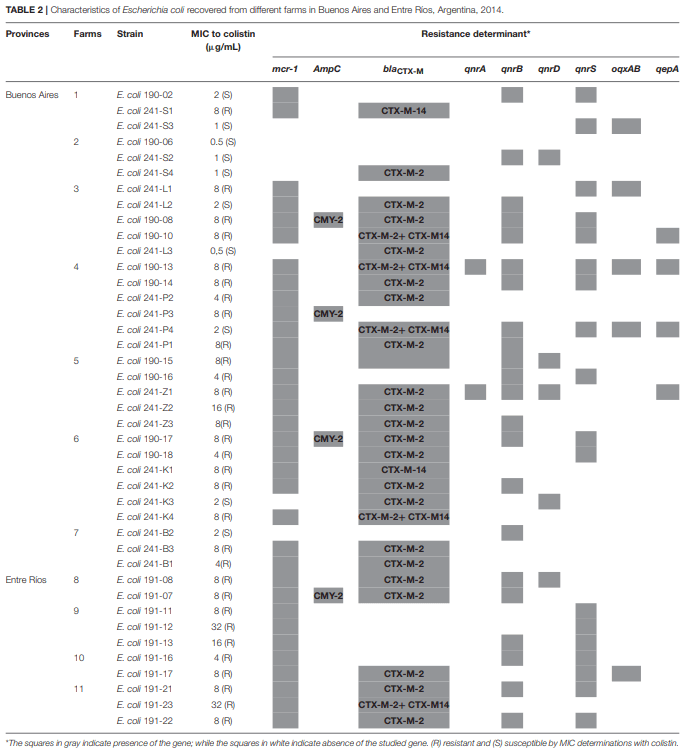
The use of antimicrobial growth promoters (AGPs) in sub-therapeutic doses for long periods promotes the selection of resistant microorganisms and the subsequent risk of spreading this resistance to the human population and the environment. Global concern about antimicrobial resistance development and transference of resistance genes from animal to human has been rising. The goal of our research was to evaluate the susceptibility pattern to different classes of antimicrobials of colistin-resistant Escherichia coli from poultry production systems that use AGPs, and characterize the resistance determinants associated to transferable platforms. E. coli strains (n = 41) were obtained from fecal samples collected from typical Argentine commercial broiler farms and susceptibility for 23 antimicrobials, relevant for human or veterinary medicine, was determined. Isolates were tested by PCR for the presence of mcr-1, extended spectrum β-lactamase encoding genes and plasmid-mediated quinolone resistance (PMQR) coding genes. Conjugation and susceptibility patterns of the transconjugant studies were performed. ERIC-PCR and REP-PCR analysis showed a high diversity of the isolates. Resistance to several antimicrobials was determined and all colistin-resistant isolates harbored the mcr-1 gene. CTX-M-2 cefotaximase was the main mechanism responsible for third generation cephalosporins resistance, and PMQR determinants were also identified. In addition, co-transference of the qnrB determinant on the mcr-1-positive transconjugants was corroborated, which suggests that these resistance genes are likely to be located in the same plasmid. In this work a wide range of antimicrobial resistance mechanisms were identified in E. coli strains isolated from the environment of healthy chickens highlighting the risk of antimicrobial abuse/misuse in animals under intensive production systems and its consequences for public health.
Keywords: Colistin, mcr-1, food-borne bacteria, Escherichia coli, CTX-M-2, qnrB, multi drug resistance
INTRODUCTION
- Penicillins: Ampicillin (AMP), Amoxicillin-Clavulanic Acid (AMC)
- Second generation cephalosporins: Cefuroxime (CXM)
- Third generation cephalosporins (TGC): Ceftiofur (CFT), Cefotaxime (CTX), Ceftriaxone (CRO), Ceftazidime (CAZ)
- Cephamycins: Cefoxitin (FOX)
- Fourth generation cephalosporins: Cefepime (FEP)
- Monobactams: Aztreonam (ATM)
- Carbapenems: Imipenem (IMI), Meropenem (MEM)
- Aminoglycosides: Kanamycin (KAN), Gentamicin (GEN), Amikacin (AMI), Streptomycin (STR)
- Tetracyclines: Tetracycline (TET)
- Quinolones: Nalidixic Acid (NAL), Ciprofloxacin (CIP), Enrofloxacin (ENR)
- Sulfonamides: Trimethoprim-Sulfamethoxazole (SXT)
- Phenicols: Chloramphenicol (CLR)
- Polymyxins: Colistin (COL)
Andres,P., Lucero,C., Soler-Bistué, A., Guerriero, L., Albornoz, E., Tran, T., et al. and Petroni,A. (2013). Differential distribution of plasmid-mediated quinolone resistance genes in clinical enterobacteria with unusual phenotypes of quinolone susceptibility from Argentina. Antimicrob. Agents Chemother. 57, 2467–2475. doi: 10.1128/AAC.01615-12
Argudín, M. A., Deplano, A., Meghraoui, A., Dodémont, M., Heinrichs, A., Denis, O., et al. (2017). Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics (Basel) 6:E12. doi: 10.3390/antibiotics6020012
Bertona, E., Radice, M., Rodríguez, C. H., Barberis, C., Vay, C., Famiglietti, A., et al. (2005). Phenotipic and genotypic characterization of resistance to third- generation cephalosporins in Enterobacter spp. Rev. Argent. Microbiol. 37, 203–208.
Botelho, L. A., Kraychete, G. B., Silva, J. L., Regis, D. V., Picão, R. C., Moreira,
B. M., et al. (2015). Widespread distribution of CTX-M and plasmid-mediated AmpC β-lactamases in Escherichia coli from Brazilian chicken meat. Mem. Inst. Oswaldo Cruz 110, 249–254. doi: 10.1590/0074-02760140389
Brenner, D. J., and Farmer, J. (2015). “Enterobacteriaceae.” in Bergey’s Manual of Systematics of Archaea and Bacteria, eds W. B. Whitman, F. Rainey, P. Kämpfer,
M. Trujillo, J. Chun, P. DeVos, B. Hedlund, and S. Dedysh, 1–24. doi: 10.1002/ 9781118960608.fbm00222
Cejas, D., Fernández Canigia, L., Quinteros, M., Giovanakis, M., Vay, C., Lascialandare, S., et al. (2012). Plasmid-Encoded AmpC (pAmpC) in Enterobacteriaceae: epidemiology of microorganisms and resistance markers. Rev. Argent. Microbiol. 44, 182–186.
CLSI (2013). “Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals; approved standard,” in CLSI Document VET01-A4, 4th Edn. (Wayne, PA: Clinical and Laboratory Standards Institute).
CLSI (2017). “Performance standards for antimicrobial susceptibility testing,” in CLSI Supplement M100, 27th Edn. (Wayne, PA: Clinical and Laboratory Standards Institute).
Cruz, G. R., Radice, M., Sennati, S., Pallecchi, L., Rossolini, G. M., Gutkind, G., et al. (2013). Prevalence of plasmid-mediated quinolone resistance determinants among oxyiminocephalosporin-resistant Enterobacteriaceae in Argentina. Mem. Inst. Oswaldo Cruz 108, 924–927. doi: 10.1590/0074-0276130084
Delgado-Blas, J. F., Ovejero, C. M., Abadia-Patiño, L., and Gonzalez-Zorn, B. (2016). Coexistence of mcr-1 and blaNDM−1 in Escherichia coli from Venezuela. Antimicrob. Agents Chemother. 60, 6356–6358. doi: 10.1128/AAC.01319-16
Diarra, M. S., Silversides, F. G., Diarrassouba, F., Pritchard, J., Masson, L., Brousseau, R., et al. (2007). Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl. Environ. Microbiol. 73, 6566–6576.doi: 10.1128/AEM.01086-07.
Dibner, J. J., and Richards, J. D. (2005). Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 84, 634–643. doi: 10.1093/ps/84.4.634 Dominguez, J. E., Figueroa Espinosa, R. A., Redondo, L. M., Cejas, D., Gutkind,
G. O., Chacana, P. A., et al. (2017). Plasmid-mediated colistin resistance in Escherichia coli recovered from healthy poultry. Rev. Argent. Microbiol. 49, 297–298. doi: 10.1016/j.ram.2017.02.001
Fernandes, M. R., Moura, Q., Sartori, L., Silva, K. C., Cunha, M. P., Esposito, F., et al. (2016). Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro Surveill. 21:30214. doi: 10.2807/1560-7917.ES.2016.21.17.30214
Ghiglione, B. (2015). Estudio de las Bases Bioquímicas y Moleculares de la Actividad Hidrolítica de Variantes Salvajes y Mutacionales de β-Lactamasas CTX-M Sobre Oximino-Cefalosporinas. Papel de mutaciones puntuales en la expansión del espectro de hidrólisis. Ph.D. thesis, Universidad de Buenos Aires.
Howarth, F., and Poulter, D. (1996). Vancomycin resistance: time to ban avoparcin.
Lancet 347:1047. doi: 10.1016/S0140-6736(96)90187-7
Huang, S. Y., Dai, L., Xia, L. N., Du, X. D., Qi, Y. H., Liu, H. B., et al. (2009).
Increased prevalence of plasmid-mediated quinolone resistance determinants in chicken Escherichia coli isolates from 2001 to 2007. Foodborne Pathog. Dis. 6, 1203–1209. doi: 10.1089/fpd.2009.0348
Jukes, T. H., Stokstad, E. L. R., Tayloe, R. R., Cunha, T. J., Edwards, H. M., and Meadows, G. B. (1950). Growth-promoting effect of aureomycin on pigs. Arch. Biochem. 26, 324–325.
Kado, C. I., and Liu, S. T. (1981). Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145, 1365–1373.
Kawanishi, M., Abo, H., Ozawa, M., Uchiyama, M., Shirakawa, T., Suzuki, S., et al. (2017). Prevalence of colistin resistance gene mcr-1 and absence of mcr-2 in escherichia coli isolated from healthy food-producing animals in japan. Antimicrob. Agents Chemother. 61:e02057-16. doi: 10.1128/AAC.02 057-16
Lai, C. C., Chuang,. Y. C., Chen,. C. C., and Tang, H. J. (2017). Coexistence of MCR-1 and NDM-9 in a clinical carbapenem-resistant Escherichia coli isolate. Int. J. Antimicrob. Agents 49, 517–518. doi: 10.1016/j.ijantimicag.2017.02.001
Lazarus, B., Paterson, D. L., Mollinger, J. L., and Rogers, B. A. (2015). Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin. Infect. Dis. 60, 439–452. doi: 10.1093/cid/ciu785
Liakopoulos, A., Mevius, D. J., Olsen, B., and Bonnedahl, J. (2016). The colistin resistance mcr-1 gene is going wild. J. Antimicrob. Chemother. 71, 2335–2336. doi: 10.1093/jac/dkw262
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Ljungquist, O., Ljungquist, D., Myrenås, M., Rydén, C., Finn, M., and Bengtsson,
B. (2016). Evidence of household transfer of ESBL/pAmpC-producing Enterobacteriaceae between humans and dogs – a pilot study. Infect. Ecol. Epidemiol. 6:31514. doi: 10.3402/iee.v6.31514
Madec, J. Y., Haenni, M., Nordmann, P., and Poirel, L. (2017). Extended-spectrum β-lactamase/ AmpC- and carbapenemase-producing Enterobacteriaceae in animals: a threat for humans? Clin. Microbiol. Infect. 23, 826–833. doi: 10.1016/j.cmi.2017.01.013
Meinersmann, R. J., Ladely, S. R., Plumblee, J. R., Cook, K. L., and Thacker,
E. (2017). Prevalence of mcr-1 in the cecal contents of food animals in the United States. Antimicrob. Agents Chemother. 61:e02244–e02216. doi: 10.1128/AAC.02244-16.
Minarini, L. A., Gales, A. C., Palazzo, I. C., and Darini, A. L. (2007). Prevalence of community-occurring extended spectrum beta-lactamase- producing Enterobacteriaceae in Brazil. Curr. Microbiol. 54, 335–341. doi: 10.1007/s00284-006-0307-z
Monte, D. F., Mem, A., Fernandes, M. R., Cerdeira, L., Esposito, F., Galvão, J. A., et al. (2017). Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 Genes in South America. Antimicrob. Agents Chemother. 61:e02718-16. doi: 10.1128/AAC.02718-16
Moore, P. R., and Evenson, A. (1946). Use of sulfasuxidine, steptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 165, 437–441. Morales, A. S., Fragoso de Araújo, J., de Moura Gomes, V. T., Reis Costa,
A. T., dos Prazeres Rodrigues, D., Porfida Ferreira, T. S., et al. (2012). Colistin resistance in Escherichia coli and Salmonella enterica strains isolated from swine in Brazil. ScientificWorldJournal 2012:109795. doi: 10.1100/2012/ 109795
Niewold, T. A. (2007). The nonantibiotic anti-Inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 86, 605–609. doi: 10.1093/ps/86.4.605
Quinteros, M., Radice, M., Gardella, N., Rodriguez, M. M., Costa, N., Korbenfeld, D., et al. (2003). Extended-spectrum β-lactamases in enterobacteriaceae in buenos aires, argentina, public hospitals. Antimicrob. Agents Chemother. 47, 2864–2867. doi: 10.1128/AAC.47.9.2864-2867.2003
Rapoport, M., Faccone, D., Pasteran, F., Ceriana, P., Albornoz, E., Petroni, A., et al. (2016). First Description of mcr-1-mediated colistin resistance in human infections caused by Escherichia coli in Latin America. Antimicrob. Agents Chemother. 60, 4412–4413. doi: 10.1128/AAC.00573-16
Rhouma, M., Beaudry, F., Thériault, W., and Letellier, A. (2016). Colistin in pig production: chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front. Microbiol. 7:1789.doi: 10.3389/fmicb.2016.01789
Rhouma, M., and Letellier, A. (2017). Extended-spectrum β-lactamases, carbapenemases and the mcr-1 gene: is there a historical link? Int. J. Antimicrob. Agents 49, 269–271. doi: 10.1016/j.ijantimicag.2016.11.026
Saba Villarroel, P. M., Gutkind, G. O., Di Conza, J. A., and Radice, M. A. (2017). First survey on antibiotic resistance markers in Enterobacteriaceae in Cochabamba, Bolivia. Rev. Argent. Microbiol. 49, 50–54. doi: 10.1016/j.ram.2016.10.002
Sennati, S., Santella, G., Di Conza, J., Pallecchi, L., Pino, M., Ghiglione, B., et al. (2012). Changing epidemiology of extended-spectrum β-lactamases in Argentina: emergence of CTX-M-15. Antimicrob. Agents Chemother. 56, 6003–6005. doi: 10.1128/AAC.00745-12
Versalovic, J., Koeuth, T., and Lupski, J. R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19, 6823 −6831. doi: 10.1093/nar/19.24.6823
Wang, J., Ma, Z. B., Zeng, Z. L., Yang, X. W., Huang, Y., and Liu, J. H. (2017). The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool. Res. 38, 55–80. doi: 10.24272/j.issn.2095-8137.201 7.024
Whang, R., Liu, Y., Zhang, Q., Jin,. L., Wang,. Q., Zhang,. Y., et al. (2018). The prevalence of colistin resistance in Escherichia coli and Klebsiella pneumoniae isolated from food animals in China: coexistence of mcr-1 and blaNDM with low fitness cost. Int. J. Antimicrob. Agents 51:739–744. doi: 10.1016/j.ijantimicag.2018.01.023
Yagi, T., Wachino, J., Kurokawa, H., Suzuki, S., Yamane, K., Doi, Y., et al. (2005). Practical methods using boronic acid compounds for identification of class C beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 43, 2551–2558. doi: 10.1128/JCM.43.6.2551-2558.2005.









.jpg&w=3840&q=75)