Introduction
Avian leucosis virus (ALV) is belonging to the family Retroviridae, subfamily ortho retrovirinae, genus Alpha retrovirus. Exogenous avian leucosis viruses(ALV) are classified into A,B,C,D and J subgroups based on their host range, cross neutralization and viral interference. They can induce different path types of neoplastic diseases in chickens (Fadly and Payne. 2003). Among these subgroups, subgroup J,A and B are more common than other subgroups in chickens(Cui et al., 2003, Du et al., 2000, Xu et al., 2004).The exogenous viruses (subgroups A, B, and J) mainly induce lymphoid leucosis (LL) and myeloid leucosis (ML) in field flocks (Fadly and Payne. 2002).No treatment exists for the exogenous viral infection. Furthermore, no commercial vaccine is available, so eradication measures are carried out by frequent detection of exogenous ALVs (Smith, et al 1998). The emergence of ALV-J forced some poultry breeders to formulate new plans to control ALV-J (Chase, 1991). Since ALV-J is spread by both vertical and horizontal transmission. The only method for blocking the vertical transmission of ALV-J is the early elimination of infected chickens (Chai. and Bates. 2006), (Chesters et al., and 2006).There are several natural modes of ALV transmission. Horizontal infection is caused either by direct contact, or contact with contaminated materials. In congenital infection, which often causes chronic viremia in poultry flocks, ALV is passed from the hen to her offspring through the chick embryo cells, and often cause chronic viremia in flocks. Finally, genetic transmission occurs when endogenous retroviruses integrate into the chicken genome and the virus spreads in a Mendelian fashion (Payne and Nair, 2012).Avian leucosis virus subgroup J (ALV-J) was first isolated from commercial broiler breeders diagnosed with myelocytomatosis (Payne et al., 1991). ALV-J appeared in the UK in the late 1980s. In the early1990s it was observed in a sporadic outbreak of myeloblastosis in Japan, disappearing after destruction of the affected stock but reappearing in 1998. By the early to mid-1990s the virus had spread to the USA, other European countries, Argentina, Israel and Taiwan, by the late 1990s to Australia and Egypt, and by the early 2000s to Malaysia and China. So the disease caused enormous economic losses in broiler breeders and broilers all over the world. This study was applied mainly on commercial meat type chickens broilers and broiler breeder chickens of various breeds in different localities in Egypt for detection of ALV antigen and antibodies using ELISA also virus isolation and molecular characterization.
Material and methods;
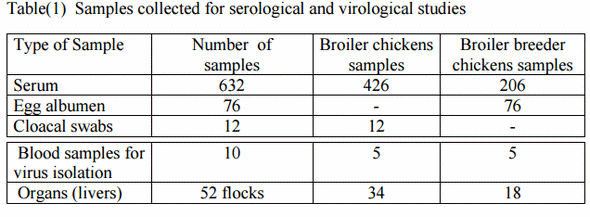
1-Samples: samples were collected from 52 flocks of commercial meat type chickens of different breeds divided to 34 flocks commercial broiler chickens and 18 flocks commercial broiler breeder chickens, located at different governorates in Egypt as detailed in table (1)
2-ELISA for detection of ALV (type A and B) and ALV-J antibodies in serum samples Commercial ELISA kits IDEXX laboratories (IDEXX Laboratories, Inc., Main, USA). Used for detection of ALV antibodies in collected serum samples.
3-ELISA for detection of ALV-p27 antigen: Commercial ELISA kits IDEXX laboratories (IDEXX Laboratories, Inc, Main, USA). Used for detection of ALV antigen in collected samples.
4-Virus Isolation on CER-Cell Culture: Organ and Buffy coat samples were prepared and inoculated on CER cell culture for ALV isolation according to (Payne & Venugopal 1999).
5-Molecularcharacterization -virus nucleic acid was extracted according to Sam Brook et al., (1989). -H5-F/ADI-R primers, for ALV from (A-E) from Metabion (Germany) and were designed according to (Mitra et al., 2013).
Results:
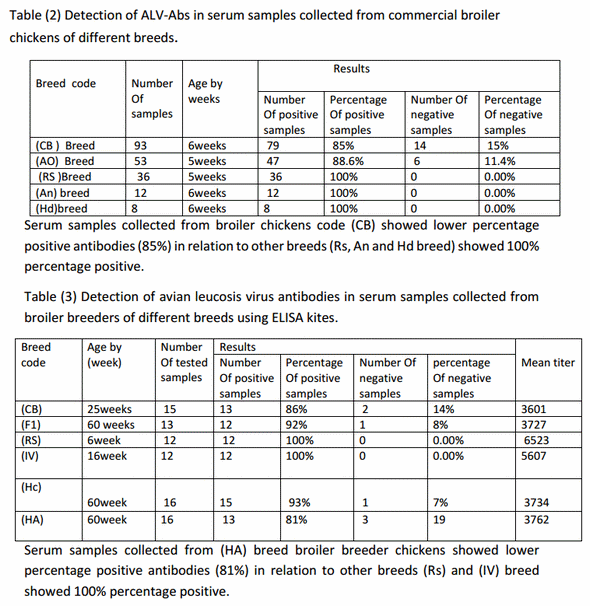
Detection of antibodies to ALV-A/Bin serum samples of commercial broiler chickens;-Among 202 serum samples 182 samples were positive for ALV-A/B by ratio of 90% and 20 samples were negative for ALV-A/B by ratio of 10% from total samples as shown in table (2).
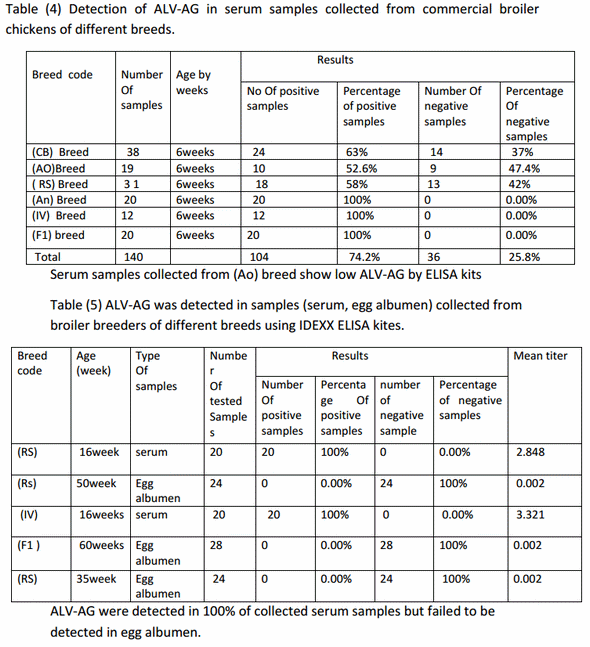
Detection of antibodies to ALV-A/B in serum samples of commercial broiler breeder chickens;-Among 84 serum samples 77 samples were positive for ALVA/B by ratio of 91.7% and 7 samples were negative for ALV-A/B by ratio of 8.3% from total samples as shown in table (3).
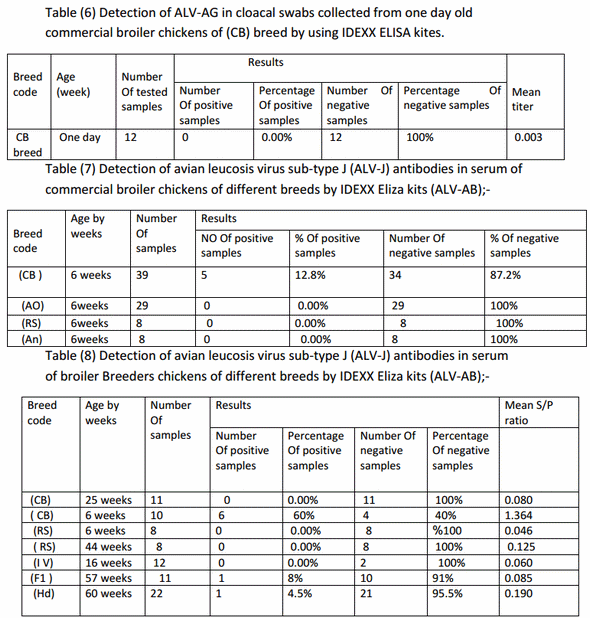
Detection of ALV-AG in serum samples of commercial broiler chickens;-Among 140 serum samples 104 samples were positive for ALV-AG by ratio of 74% and 36 samples were negative for ALV-AG by ratio of 26% from total samples as shown in table (4).
Also, 12 cloacal swabs of one-day old broiler chicks show 100% negative result as shown in table (5).
Detection of ALV-AG in serum and egg albumin samples of commercial broiler breeder chickens;-
40 serum samples were tested show 100% positive result for ALV-AG. While 76 egg albumin samples were tested show 100% negative result as shown in table (6).
Detection of antibodies to ALV- J in serum samples of commercial broiler chickens;-
Among 84 serum samples 5 samples were positive for ALV- J by ratio of 6% and 79 samples were negative for ALV- J by ratio of 94% from total samples as shown in table (7).
Detection of antibodies to ALV- J in serum samples of commercial broiler breeder chickens;-
Among 82 serum samples 8 samples were positive for ALV- J by ratio of 9.7% and 79 samples were negative for ALV- J by ratio of 92.3% from total samples as shown in table (8).
Detection of ALV in liver samples by PCR test;-
5 liver samples from 6 weeks broiler chickens were tested by conventional PCR the 5 samples show negative result.
Detection of ALV in blood by PCR test;-
10 blood samples from 6weeks broiler chickens were collected for Buffy coat separation, then pooled in 2 samples and tested by PCR, the result revealed one positive and one negative
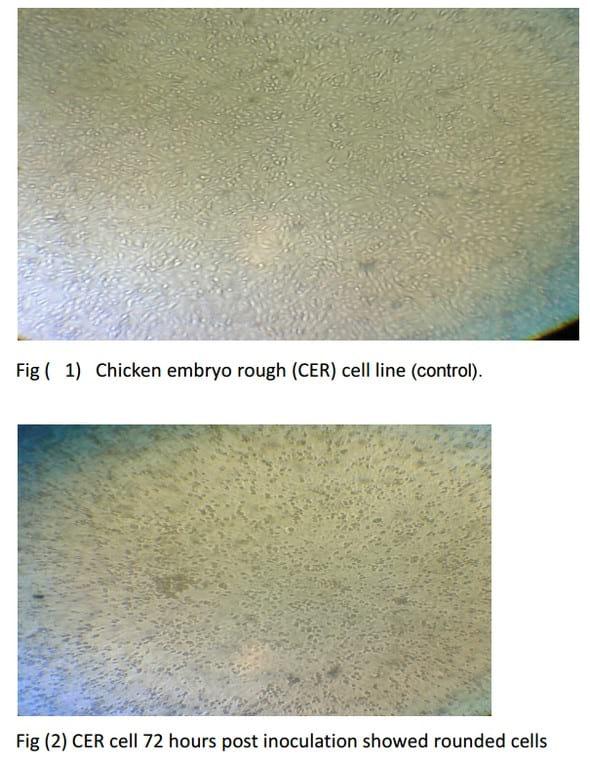
Isolation of ALV on CER cell line
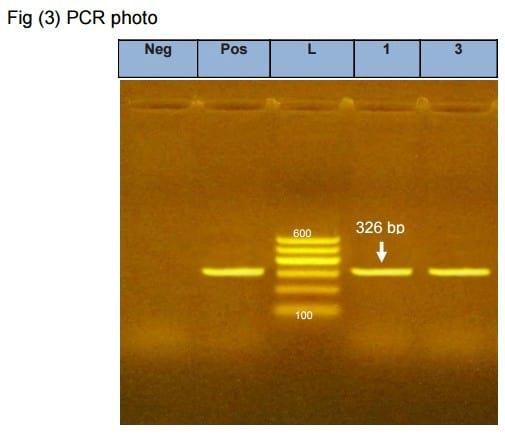
Liver samples (2 samples) and one Buffy coat sample were taken from suspected flocks and inoculated on CER culture, after 72 hours the 3 samples showed CPE which become clear after 3rd passage in the form of cell rounding and cell aggregation. (Fig 2), then the harvested virus was detected using PCR (Fig 3).
Photo (1). PCR results for the env gene of Avian Leukosis virus (ALV). Negative control show negative amplification of the specific segment, while lane 1, lane 2 and Positive control show positive amplification of the 326 bp of the env gene. L [100 bp ladder (Qiagen, 100-600 bp)].
*1-Buffy coat sample from 6weeks broiler chickens propagated on CER cell line then detected by PCR.
2- Tissue sample from broiler breeder chickens propagated in CER cell line then detected by PCR.
Discussion
Avian leucosis virus ALV is a retrovirus that induces malignant neoplasm in poultry and has been reported worldwide to be a major cause of serious economic losses to the poultry industry. Negative impact of the disease on chickens includes reduced growth, unevenness of growth rate. Within flocks, and a greater susceptibility to developing serious disease when challenged by immunosuppressive viruses or secondary bacterial invaders (Bagust et al., 2004).
Lymphoid leucosis induced by subgroups ALV-A and ALV-B is one of the most common tumor diseases in chickens (Payne and Nair 2012)
At the past, most of the reports have been confirmed that ALV type A and B spread only in egg type chickens causing lymphoid leucosis and only a few reports have been confirmed that meat-type chickens can also be infected by ALV subgroup A and B (Li et al.,2009).
So this study was applied only on commercial meat-type chickens (broiler breeders and broiler chickens) and gives high positive result by ELISA tests for detection of ALV antibodies for type A and B reach to 91.7% positive in broiler breeders as average of all breeds while Serum samples collected from broiler breeder chickens code (HA) showed lower percentage positive antibodies (81%) in relation to other breeds showed 100% percentage positive.
Also, positive result by ELISA tests for detection of ALV antibodies for type A and B reach to 90% in broiler chickens as average of all breeds while Serum samples collected from broiler chickens code (CB) showed lower percentage positive antibodies (85%) in relation to other breeds showed 100% positive. Also, commercial ELISA kits aimed to antigen is usually the first choice for the identification of ALV in the poultry industry but it is dependent on the group specific antigen p27 which is shared by both exogenous and endogenous ALV (Chesters et al., 2002).
ELISA is useful for the detection of ALV group-specific antigen (p27) which is common to all of the subgroups. It is a sensitive, safe, rapid and easy to perform diagnostic tool (Smith et al., 1979). The ALVELISA diagnostic method is reported to have 99.2% sensitivity and 100% specificity, and can be used clinically for screening purpose (Pham et al., 1999; Silva et al., 2007). For there is no commercial vaccine to control ALV up to now, it is reasonable to reflect the ALV infection status by serological methods so in this study the positive result by ELISA tests for detection of ALV antigen reach to 100% positive result of collected serum samples from broiler breeders but failed to be detected in egg albumen. And to 74.2% positive result in broiler chickens while Serum samples collected from breed code (AO)show low ALV-AG by ELISA kits 52%.
For another important sub-group, ALV-J has been identified and reported from meat-type breeders and commercial broilers it causes both late-onset myeloid tumors and acute tumors even in 4-5 weeks old birds (fadly and smith,1999).
Our study show that Among 84 serum samples 5 samples were positive for ALV- J by ratio of 6% in broiler chickens, Detection of antibodies to ALV- J in serum samples of commercial broiler breeder chickens show that Among 82 serum samples 8 samples were positive for ALV- J by ratio of 9.7%.
As ALV-J virus transmitted by two methods horizontal and vertical transmission, and IDEXX ELIZA kits for ALV-J (AB) detect only the antibodies due to horizontal infection and don’t detect antibodies of vertical transmission infection giving negative results, so the high negative percent in broilers 94% and 90.3% in broiler breeders indicate that the vertical transmission is the most common route than horizontal route as mode of transmission of ALV-J virus. These results confirmed by virus isolation CER cell line tissue culture, then testing of cultures by PCR test for lymphoid leucosis (A-E) which give positive results for presence of avian leucosis virus.
Our results concluded that the vertical transmission route of infection is the most common route for ALV-J virus. Our recommendations are screening of breeder chickens for ALV and removal of chickens found to be viraemic, may also help in controlling the spread of ALV from dam to chicks as the virus is known to be transmitted vertically. Also, efforts should be channeled into developing vaccines that will protect birds against the effect of this important group of retroviruses of chickens.
References;
1-.Bagust, T., Fenton, S. and Reddy, M. (2004); avian leucosis virus sub-group J (ALV-J): Developing laboratory technologies for diagnosis in Australia. Rural Industries Research and Development Corporation, Publication No 04/116.
2- Chai, N., and P. Bates. (2006); Na_/H_ exchanger type 1 is a receptor for pathogenic subgroup J avian leucosis virus. Proc. Natl. Acad. Sci. U. S. A. 103:5531–5536.
3-Chase, W. B. (1991); Eradication of avian leucosis virus by breeder companies: results, pitfalls and cost benefit analysis, p. 5–7. In D. Swayne and D. Zander (ed.), Proceedings of the Avian Tumor Virus Symposium. American Association of Avian Pathologists, Kennett Square, PA.
4-Chesters, P.M., K. Howes, L. Petherbridge, S. Evans, L.N. Payne and K. Venugopal, (2002); The viral envelope is a major determinant for the induction of lymphoid and myeloid tumors by avian leucosis virus subgroups A and J, respectively. J. Gen. Virol., 83:2553-2561 .
5-Chesters, P. M., L. P. Smith, and V. Nair. (2006); E (XSR) element contributes to the oncogenicity of avian leukosis virus (subgroup J). J. Gen. Virol. 87:2685–2692.
6- Cui, Z., Y. Du, Z. Zhang, and R. F. Silva. (2003); Comparison of Chinese field strains of avian leukosis subgroup J. viruses with prototype strain HPRS-103 and United States strains. Avian Dis. 47:1321–1330
7-Du, Y., Z.Z. Cui, A. Qin, R. Silva and L. Lee, (2000); Isolation of subgroup J avian leucosis viruses and their partial sequence comparison. Chin. J. Viro., 16:341-346.
8-Fadly, A.M. and E.J. Smith, (1999); Isolation and some characteristics of a subgroup J-like avian leucosis virus associated with myeloid leucosis ALV-J strain NX0101 decreases HI antibody titers to NDV in meat-type chickens in the United states. Avian Dis., 43:391-400
9-Fadly, A. M., and L. N. Payne. (2002); Leukosis/sarcoma group, p. 465–516. InY. M. Saif, H. J. Barnes, A. M. Fadly, L. R. McDougald, D. E. Swayne, and J. R. Glisson (ed.), Diseases of poultry, 11th ed. Iowa State University Press, Ames, IA.
10-Fadly, A.M., T.F. Davison, L.N. Payne and K. Howes(1989) ;Avian leucosis virus infection and shedding in brown leghorn chickens treated with corticosterone or exposed to various stressors. Avian Pathol. 18:283-298.
11-Fadly A.M., and Payne, L.N., (2003); Leucosis / Sarcoma group. In: Diseases of poultry. 11th ed. Saif, Y.M.; (eds). Iowa state Univ. press, Ames, Iowa; PP 465-516.
12-Li, X., K. Shi and M. Luo (2009); a. Diagnosis of avian leucosis subgroup J infection in breeding chicken. Avian Dis., 21: 13-17.
13- L.N. Payne & K. Venugopal. (2000); neoplastic diseases: Marek's disease, avian leukosis and Reticuloendotheliosis. Rev. Sci. tech. Off. Int. cpiz. 19 (2), 544-564.
14-Mitra, N.; Verma, R.; and Singh, A. (2013): Early Detection of Avian Oncogenic Viruses from Blood of Apparently Healthy Chickens. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, March 2013, Volume 83, Issue 1, pp 53-58.
15-Payne, L.N., Nair, V (2012.); the long view: 40 years of avian leukosis research. AvianPathology 41 (1), 11–19.
16-Payne, L. N., S. R. Brown, N. Bumstead, K. Howes, J. A. Frazier, and M. E. Thouless. (1991). A novel subgroup of exogenous avian leukosis virus inchickens. J. Gen. Virol. 72:801– 807.
17-Pham, T.D., Spencer, J.L. and Johnson, E.S. (1999); Detection of avian leukosis virus in albumen of chicken eggs using reverse transcription polymerase chain reaction. Journal of Virology Methods, 78: 1-11.
18-Sambrook, J.; Fritscgh, E.F.;andMentiates (1989): Molecular coloning. A laboratory manual.Vol! Cold spring Harbor Laboratotry press, New York.
19- Silva, R.F., Fadly, A.M. and Taylor, S.P. (2007).Development of a polymerase chain reaction to differentiate avian leucosis virus (ALV) subgroups: Detection of an ALV contaminant in commercial Marek's disease vaccines. AvianDiseases, 51: 663-667.
20-Smith, E. J., Fadly, A. M. and Okazaki, W. (1979); an enzyme-linked immunosorbent assay for detecting avian leukosis-sarcoma viruses. Avian Diseases, 23: 698-707.
21-Smith, L. M., S. R. Brown, K. Howes, S. McLeod, S. S. Arshad, G. S. Barron, K. Venugopal, J. C. McKay, and L. N. Payne. 1998. Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus. Virus Res. 54:87–98.
22-Xu, B., W. Dong, C. Yu, Z.He and Y. Lv et al.,( 2004);Occurrence of avian leucosis virus subgroup J in commercial layer flocks in china. Avian Pathol. 33:13-17









.jpg&w=3840&q=75)