Introduction
Chronic Respiratory Syndrome (CRS) in hens is an infectious, contagious, chronic disease that causes great economic losses worldwide in the poultry industry(rose et al., 2003, Burch, 2009). In Cuba, in 2005 evidence was shown that this syndrome constitutes 20 percent of the infectious contagious diseases affecting laying hens (Colas et al., 2006). The presentation of this disease involves several factors, which are environmental and handling factors, as well as bacterial and viral agents that can trigger and worsen this respiratory process(Guadalupe, 2005).
The bacterial agents associated with the CRS include, mainly: Avibacterium paragallinarum (a. paragallinarum), Pasteurella multocida (p. multocida), Gallibacterium anatis (g. anatis), Ornithobacterium rhinotracheale (o. rhinotracheale), Staphylococcus spp., Escherichia coli (e. coli) and Mycoplasma gallisepticum (M. gallisepticum) as the main agent(Kleven, 2000 ;) Ricci, 2007, Cerdá, 2009). However, in recent years, the presence of serovars of the emerging pathogen O. rhinotracheale, associated to CRS, has also been reported (Icochea, 2009). Viral agents associated with this syndrome are the Newcastle disease virus (NC), the Avian Influenza, the Avian Infectious Bronchitis (IB), and the Avian Metapneumovirus, which combine with bacterial agents and cause a more severe clinical pathological manifestation (Dave, 2003).
In Cuba, there are programs of immunization against IB and the NC disease, with the application of live vaccines to layers and inactivated vaccines in breeders (Viamontes, 2006). On the other hand, bacteria control is done via implementation of biosafety measures and practices, which constitute a very useful tool in the prevention and control of endemic and exotic diseases (Merino, 2009). These measures include serological surveillance: rapid sero-agglutination on plate (SAR for M. gallisepticum), inhibition of hemo-agglutination (IHA for NC and IA) and the ELISA tests for IB (Masdeu et al., 1997; Rosado, 2001). Taking into consideration the geographical distribution and incidence in the region, as well as the economic losses caused by these pathogens in production, we set out to evaluate the serological response in layers with chronic respiratory syndrome before exposure to Ornitobacterium rhinotracheale, associated to other agents.
Materials & Methods
120 White Leghorn laying hens were selected from a layer replacement unit in the Artemisa province, Cuba, 12 weeks of age, identified by wing bands. They were relocated at week 16 to a layer unit with various ages and a record of respiratory infection. The food given was balanced feed according to the specifics and they were vaccinated according to the immunization program existing in Cuba (Technical instructions, "Layer and its replacements. Breeding technology". from 2007). The IB vaccine was applied (H120 strain) and the NC (La Sota strain) on days 1, 35 and 85, and 7, 49, 90 and 228, respectively, reflected in the Technical Instructions "layer and its replacements. Breeding technology". from 2007).
Blood samples were taken monthly from week 12 until week 50, and serum was collected, according to the methodology described by FAO (2004).
The serological study to demonstrate, indirectly, the presence of birds reacting to O. rhinotracheale and M. gallisepticum was plannedup to 38 weeks of age and extended up to week 50 for birds reacting to the IB and NC viruses.
Flock Chek company commercial kits were used for O. rhinotracheale and IB, respectively; the kits were used according to the manufacturer''''''''''''''''s instructions. Samples were considered positive if they showed titles equal or higher than 396 in the case of IB and, starting from the coefficient of positive samples (CMP), equal or higher than 0.4 for O. rhinotracheale.
For rapid sero-agglutination on plate (SAR), colored antigen of M. gallisepticum, marketed by Laboratorios Intervet was used and its application followed the manufacturer's instructions. For the detection of Ac against NC titles, hemo-agglutination inhibition (IHA) was applied, according to the methodology described by FAO (2004).
The statistical package COMPROP-1 was used to compare the proportions of birds testing positive to Ornitobacterium rhinotracheale,Mycoplasma gallisepticum, and for birds reacting to IHA titles higher than 1/8 to NC, and the STATGRAPHICS PLUS 5.1 package was used for simple variance analysis of the geometric means of the of antibodies titles to Avian Infectious Bronchitis.
Results and Discussion
Among the different segments of the production chain of layers and their replacements in Cuba, respiratory infections occur most frequently in the layer category, i.e. after 16 weeks of age (Boado et al., 1991).
In this study, we found birds testing positive to M. gallisepticum and O. rhinotracheale (Figure 1 and 2), which suggests the presence of an infection by both microorganisms, because the birds were not subjected to immunization programs specific against these diseases. In the case of birds testing positive to M. gallisepticum, from week 16 and up to 25 weeks of age, an increase in the number of birds testing positive to this microorganism, differing significantly from the rest of the weeks analyzed was observed. Similar findings have been published by Rosado, (2001), who demonstrated, using the ELISA technique, a high circulation of M. gallisepticum in laying hens affected by CRS in all provinces of Cuba.
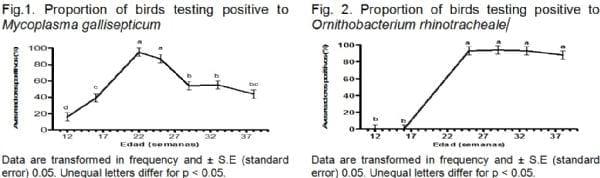
As seen in Figure 2, in the two initial samplings corresponding to weeks 12 and 16, the serological response to O. rhinotracheale shows birds that are not testing positive to infection by this microorganism. Evidence of the persistent significant increase in the proportion of birds testing positive from week 25 until week 38, obtained in this study, is very similar to results reported by Cerdà et al. (2010), in sero-epidemiologic studies with O. rhinotracheale performed in the United States and Germany.
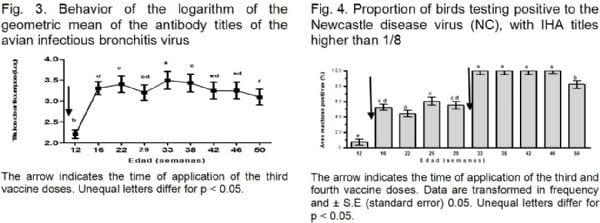
In order to find out about the protection status or evidence of IB infection, a kinetics study at different post-vaccination times is performed, for the purpose of controlling this disease in intensive production in Cuba. This is done taking into consideration two fundamental elements: Immunizations at the layer breeding replacement stage and checking the layers serological response (Noda et al., 2002 ;) Neck et al., 2004). Geometric averages of Ac to IB titles (Figure 3) revealed that from the third vaccination on, an increase in titles was observed; starting at 16 weeks, reaching a peak at 22 weeks, which coincides with what has been said by Cavanagh (2003), that several applications of the IB vaccine are much more efficient and what has been said by Acevedo et al. (2010), that the titles may be kept for a Langer period of time and start declining three months after vaccination.
However, another increase in Ac titles against IB titles was observed in week 33, in asymptomatic birds. This behavior may be related to infection by a different viral strain and not to the persistence of the vaccine virus; since, coincidentally, a week earlier, in a different batch of birds, aged 9 to 10 months, a case of CRS appeared, in which a different strain of the IB virus was isolated and molecularly identified, which may differ from the vaccine strain (Acevedo et al., 2010). Valencia (2007) points out that variants of the IB virus exist, which continue to appear and many of them are circulating in healthy chicken all the time.
Figure 4 shows the proportion of birds testing positive with Ac IHA titles against NC higher than 1: 8. This is evidence that birds testing positive do not correspond with a possible viral NC infection.
Conclusions
This study made it possible to observe for the first time the presence of birds testing positive to O. rhinotracheale, and confirm the presence of M. gallisepticum, among laying hens affected by chronic respiratory syndrome. The serological study in poultry vaccinated against IB showed a sero-conversion which could be related to a circulation of this agent in the examined population, which constitutes a potential danger in poultry farming. The evaluation of birds vaccinated against NC showed no IHA titles corresponding to an infection, but there was an adequate post-vaccination response.
Bibliography
Acevedo AM, Burgher Y, Colas M; Relova D, Correa A, Bacallao E, Noda J. 2010. Detección en muestra clínica e identificación de aislados del virus de la Bronquitis Infecciosa Aviar por un ensayo de Reverso Transcripción acoplado a Reacción en Cadena de la Polimerasa. Rev. Salud Anim. 32(2):12-117.
Boado E, Laurent R, Herrera C, Quintero D, Canovas A. 1991. Prevalencia de las principales enfermedades en las diferentes categorías de aves durante las épocas del año. Rev. Cub. Cienc. Avíc. 18(3):257-262.
Burch D. 2009. Revisión de la actividad del tiamulin contra el Mycoplasma spp y su uso en la prevención de la transmisión vertical en reproductoras ponedoras. pp. 394-398. En: Memoria del XXI Congreso latinoamericano de avicultura. Oct 6-10; Ciudad de la Habana, Cuba.
Cavanagh D. 2003. Survey acute respiratory syndrome vaccine development: Experience of vaccination. Avian Patholog. 32(6):567-582.
Cerdá R. 2009. Interpretación de los resultados del diagnóstico de micoplasmas aviares. pp. 353-357. En: Memoria del XXI Congreso latinoamericano de avicultura; Oct 6-10; Ciudad de la Habana, Cuba.
Cerdá R, Uriarte J, Origlia J, Petruccelli M. 2010. Ornithobacterium rhinotracheale: Su importancia como patógeno respiratorio en pollos y su asociación con Mycoplasma synoviae UTL:http://www.vetanco.com.ar/pdf/ornithobacterium_rhinotracheale_capia.pdf. Acceso: 16-Oct-2010.
Colas M, Santana Y, Merino A, Mojena K, Correa A. 2006. Frecuencia de presentación de las principales enfermedades ponedoras White Leghorn. Rev. Cub. Cienc. Avíc. 30(2):103-106.
Cuello S, Noda J, Alfonso P, Perera CL. 2004. Bronquitis Infecciosa Aviar. Cinética de anticuerpos postvacunales en reproductoras y su transferencia a la progenie. Rev. Salud Anim. 26(1):42-47.
Dave C. 2003. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 32(6):567-582.
FAO. 2004. Chapter 3. Vaccination. A Technology Review: Newcastle disease. p.21-27.
Guadalupe A. 2005. Implementación del Diagnostico de Mycoplasma gallisepticum y Mycoplasma synoviae mediante PCR- RFLP en aves de producción [Tesis en opción al grado de Maestro en Ciencias Veterinarias con especialidad en Salud Animal]. Universidad Autónoma de Nuevo León.
Icochea E. 2009. Diagnóstico diferencial de enfermedades respiratorias en aves bajo condiciones de campo y de laboratorio. pp. 413-420. En: Memoria del XXI Congreso latinoamericano de avicultura; Oct 6-10; Ciudad de la Habana, Cuba.
Kleven SH. 2000. Micoplasmosis. En: 1er Seminario de Actualización en Patología Aviar. Merial-Cerval. Georgia, USA.
Masdeu V, Acosta I, Rejo T, Espinosa V, Herrera C, Hernat R, Díaz G. 1997. Características de los procesos respiratorios mediante el diagnóstico serológico y el cuadro clínico lesional. Rev. Cub. Cienc. Avíc. 21(1):11-16.
Merino LA. 2009. Conferencias de Bioseguridad. Curso de Patología y Epizootiología Aviar. Folletos pp. 1-30.
Noda J, Cuello S, Alfonso P, Casañas J. 2002. Bronquitis infecciosa aviar: Obtención de antígeno y detección de anticuerpos por inhibición de la hemoaglutinación. Rev. Cub. Cienc. Avíc. 26 (2):89-95.
Ricci M. 2007. Prevención y control de los miscoplasmas aviares. Avic. Prof. 25(2):16-18.
Rosado I. 2001. Obtención y evaluación de un candidato vacunal contra la infección por Mycoplasma gallisepticum. [Tesis en opción al grado científico de Doctor en Ciencias) - Universidad Agraria de la Habana]. Centro Nacional de Sanidad Agropecuaria.
Rosado I, Ruedas D, Sánchez L, Acosta I, Véliz T, Lobo E, et al. 2003. Evaluación de la eficacia en condiciones controladas de una bacterina oleosa contra Mycoplasma gallisepticum obtenida en fermentadores de 7L. Rev. Cub. Cienc. Vet. 28(1):43-45.
Valencia B. 2007. Bronquitis Infecciosa: Soluciones prácticas. En: Memoria Congreso Nacional de Avicultura. URL:http://www.amevea-ecuador.org/memorias.htm. Acceso: 16 febrero, 2010.
Viamontes O. 2006. Impacto económico de una adecuada estrategia de inmunización contra la enfermedad de Newcastle. p. 474. En: Memorias V congreso de avicultura.