Introduction
The intestinal epithelium constitutes the largest and most important barrier against external environmental agents and has three critical functions: 1) To prevent the entry of harmful intraluminal microorganisms, antigens, and toxins; 2) To enable the selective translocation of dietary nutrients and electrolytes into circulation; and 3) To tolerate the beneficial microbiome [1–4]. Inappropriate immunological reactions against food compounds, such as lactose or gluten, can lead to the breakdown of oral tolerance and the development of intestinal immune disorders in humans [5–16] and several investigators have described how the composition of the diet, also has a tremendous impact in digestibility and gut health of [17–19]. Thousands of years of evolution shaped the digestive system of the jungle fowl and wild pig to deal with the dietary ingredients they encounter in an efficient manner. More recently, throw intensive genetic manipulation, nutrition and health programs we have modified the biology and growth potential of production animals. Today, modem commercial monogastric animals diets contain 2 or 3 ingredients that may constitute >75% of intake. Corn is usually the main source of energy in poultry diets, but at times it is difficult to formulate least cost diets using corn, therefore, unconventional grains have to be used. Specifically, rye-based diets versus traditional corn-based diets, were different cereals are used as principal source of energy. The inclusion of rye in poultry diets has been fraught with problems, principally related to the production of sticky droppings, malabsorption syndrome, elevated feed conversion and intestinal bacterial overgrowth [20–22]. The endosperm cell wall of rye and wheat is comprised mainly of highly branched arabinoxylans which increase the viscosity of the digesta [23]. Elevated viscosity reduces digestibility and performance by interfering with the movement of particles and solutes across the intestinal lumen [24] favoring intestinal bacterial overgrowth [22]. The purpose of the present study was to evaluate the use of rye on bacterial translocation (BT), intestinal viscosity, microbiota composition, and bone mineralization when compared with a traditional cereal (corn) in turkey poults.
Material and Methods
Animal source, diets and experimental design
In order to show that the same or similar results can be achieved independently, two independent experiments were conducted in the present study. In each experiment, forty turkey poults were obtained from a commercial hatchery (Cargill Gentry, AR, USA), randomly assigned to 2 groups (n = 20 turkey poults), and placed into isolator chambers in a controlled age-appropriate environment with unrestricted access to feed and water for 10 days. Turkey poults received either a corn or rye diet meeting the nutritional requirements of poultry recommended by National Research Council [25]. Diets were antibiotic-free (Table 1). All animal handling procedures were in compliance with Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas. Specifically, the IACUC approved this study under the protocol #11047- “Evaluation of direct fed microbials and prebiotics in poultry”. At ten days of age, in both experiments, 12 turkey poults in both treatment groups were randomly selected, and given an oral gavage dose of fluorescein isothiocyanate dextran (FITC-d; 2.2mg/mL/bird; MW 3,000–5,000 Da; Sigma Aldrich Co., St. Louis, MO). After 2.5 h they were humanly killed using carbon dioxide asphyxiation method. Blood samples were collected from the femoral vein for measuring leakage of FITC-d. Liver was collected to evaluate BT. Duodenal, ileal, and cecum gut sections were collected to enumerate bacteria. For intestinal viscosity, 5 turkey poults from each group ere humanly killed and intestinal digesta were individually collected. Additionally, tibias were collected for bone parameters as describe below.
Viscosity
Total intestinal digesta contents were collected from Meckel's diverticulum to the ileocecocolonic junction. For viscosity analysis, approximately 1.5 g (wet weight) of the fresh digesta was immediately placed in a microcentrifuge tube and centrifuged at 12,000 x g at 4°C for 5 min. The supernatant fluid was collected and stored on ice until viscosity measurement was determined using a LVDV-I Brookfield digital cone-plate viscometer fitted with a CP-40 spindle (Brookfield Engineering, Middleboro, MA). The analyzed samples and the viscometer cup were maintained at 40°C during viscosity measurement. Viscosity was measured in centipoise (cP = 1/100 dyne s/cm2 ) and the results were reported as log10 cP.
Bacterial translocation
Briefly, the right half of the liver was removed from each turkey poults, collected in sterile bags, homogenized, weighed and 1:4 wt/vol dilutions were made with sterile 0.9% saline. Ten-fold dilutions of each sample, from each group were made in a sterile 96 well Bacti flat bottom plate and the diluted samples were plated on MacConkey Agar (VWR Cat. No. 89429–342 Suwanee, GA 30024) to evaluate total counts of Enterobacteriaceae per gram of tissue as has been previously described [11,26,27].
Serum determination of FITC-d
Blood was kept at room temperature for 3 h and centrifuged (1,000 x g for 15 min) to separate the serum from the red blood cells. FITC-d levels of undiluted serum were measured at excitation wavelength of 485nm and emission wavelength of 528nm (Synergy HT, Multi-mode microplate reader, BioTek Instruments, Inc., Vermont, USA). Fluorescence measured was then compared to a standard curve with known FITC-d concentrations. Gut leakage for each bird was reported as μg of FITC-d/mL of serum.
Enumeration of bacteria
Whole duodenum, ileum, and both ceca were aseptically removed, separated into sterile bags, and homogenized. Samples were weighed and 1:4 wt/vol dilutions were made with sterile 0.9% saline. Ten-fold dilutions of each sample, from each group were made in a sterile 96 well Bacti flat bottom plate and the diluted samples were plated on three different culture media; to evaluate total number of lactic acid bacteria (LAB) in Man Rogosa Sharpe (Difco Lactobacilli MRS Agar VWR Cat. No. 90004–084 Suwanee, GA 30024); total Enterobacteriaceae in MacConkey; and total anaerobes in tryptic soy agar with sodium thioglycolate plates (TSA, catalog no. 211822, Becton Dickinson, Sparks, MD).
Bone parameters
Bone parameters were measured according to the methods as described by Zhang and Coon, [28]. Tibias from each turkey poults were cleaned of attached tissues. Bones from the left leg were subjected to conventional bone assays and tibia from the right leg was used to determine breaking strength. The bones from left tibia were dried at 100°C for 24 h and weighed again. The samples were then incinerated in a muffle furnace (Isotemp muffle furnace, Fisher Scientific, Pittsburgh, PA) at 600°C for 24 h in crucibles. Finally, the content of calcium and phosphorus in the tibia was determined using standard methods [29] and were reported as percentage of dry matter. The right tibial diaphyses from individual birds were cleaned of adherent tissues, the periosteum was removed, and the biomechanical strength of each bone was measured using an Instron 4502 (Norwood, MA) material testing machine with a 100 kg Load Cell. The bones were held in identical positions and the mid-diaphyseal diameter of the bone at the site of impact was measured using a dial caliper. The maximum load at failure was determined using a three-point flexural bend fixture with a total distance of 30 mm between the two lower supporting ends. The load, defined as force in kilograms per square millimeter of crosssectional area (kg/mm2 ), represents bone strength. The rate of loading was kept constant at 20 mm/min collecting 10 data points per second. The data were automatically calculated using Instron’s Series IX Software (Norwood, MA).
Statistical analysis
All data were subjected to one-way analysis of variance as a completely randomized design using the General Linear Models procedure of SAS [30]. Data are expressed as mean ± standard error. Significant differences among the means were determined by using Duncan’s multiple-range test at p<0.05.
Results
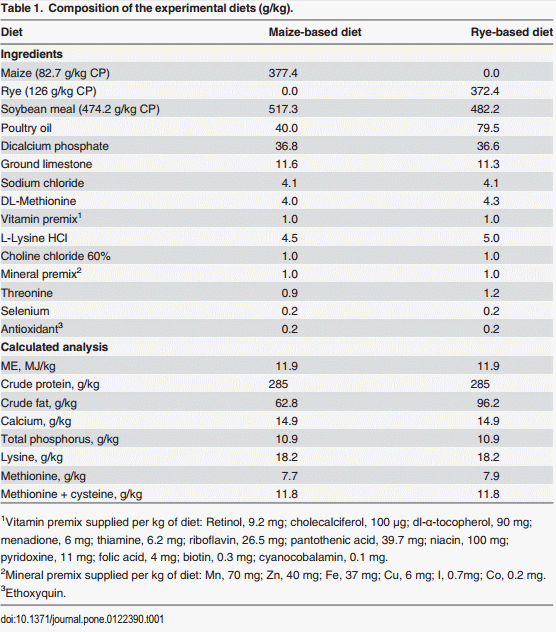
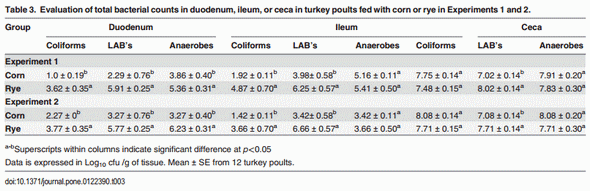
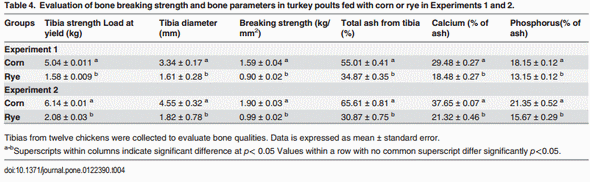
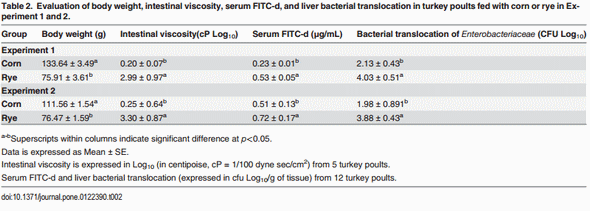
The evaluation of body weight, intestinal viscosity, serum FITC-d, and liver BT in turkey poults fed with a corn diet or a rye diet of Experiment 1 and 2 are summarized in Table 2. A significant (p<0.05) reduction in body weight was observed in turkey poults fed with rye as compared with corn in both experiments. However, turkey poults fed with rye showed an increase in intestinal viscosity which was associated with elevated (p<0.05) serum FITC-d, and an increase in BT of Enterobacteriaceae to the liver (Table 2). Total bacterial counts in duodenum, ileum, and ceca of neonatal turkey poults fed with a corn or rye diet in Experiment 1 and 2 are summarized in Table 3. In both trials, turkey poults that were fed with rye had a significant increase in the number of total LAB that were observed in duodenum, in ileum, and ceca when compared with turkey poults fed with corn. In these turkey poults, a significant increase in the total number of coliforms was also observed in duodenum and ileum but not in cecum, whereas, an increase in total number of anaerobes was observed only in the duodenum (Table 3). The results of the evaluation of bone breaking strength and bone parameters in neonatal turkey poults fed with corn or rye in Experiments 1 and 2 are summarized in Table 4. Significant increases in tibia diameter, tibia breaking strength, tibia ash, and calcium and phosphorus percentages were observed in turkey poults that fed corn when compared with turkey poults that fed the rye diet (Table 4).
Discussion
Feeding cereals high in non-starch polysaccharides (NSPs) leads to increased feed conversion ratios and lower body-weight gain [31–33]. In addition, high NSPs diets have also been associated with Necrotic Enteritis (NE), a multi-factorial disease caused by Clostridium perfringens that is probably the most important bacterial disease in terms of economic implications in turkey poults [24,34,35]. Since poultry has little or no intrinsic enzymes capable of hydrolyzing these NSPs, exogenous xylanases as additives are used in an attempt to reduce this anti-nutritive factors [21,23,36]. Several mechanisms of action of NSPs on nutrient absorption have been described that include an increased digesta viscosity, thickening of the mucous layer on the intestinal mucosa, epithelial cell apoptosis, inflammation in response to the dysbacteriosis caused by a higher soluble NSPs content [30,37–39], and the nutritional and economic consequences of mounting an inflammatory response in poultry is inversely related to body-weight gain and overall performance [22,40]. In the present study, turkey poults fed with rye showed an increase in intestinal viscosity, elevated BT of Enterobacteriaceae, and increased serum FITC-d (Table 2). These changes were also associated with significant bacterial overgrowth when compared with turkey poults fed with corn (Table 3). It is important to mention that the total bacterial counts were evaluated on selective media and using standard microbiological procedures that are known to severely under-represent the true microbiological diversity. Nevertheless, it provides interesting results that justify further exploration using metagenomics analysis. Variations in the composition of the microbiome within different segments of the alimentary tract are influenced by the environment, by the diet, and by the host [41–44]. Alterations in gut permeability are associated with BT in the portal and/or systemic circulation in several types of leaky gut syndromes leading to systemic bacterial infections [26,45]. Similarly, FITC-d is a large molecule (3–5 kDa) which does not usually leak through the intact gastrointestinal tract barrier. However, when conditions disrupt the tight junctions between epithelial cells, the FITC-d molecule can enter circulation as demonstrated by an increase in trans-mucosal permeability associated with chemically induced disruption of tight junctions by elevated serum levels of FITC-d after oral administration [46]. The significant reduction in bone strength and mineralization (Table 4) confirmed previous studies that have shown that high NSPs diets in poultry or gluten intolerance in humans, are also associated with malabsorption of minerals and fat-soluble vitamins [13,47–51]. Performance of rye-fed birds can be improved markedly by dietary supplementation with exogenous xylanase [21,52,53]. Previously, we have reported that dietary inclusion of selected Direct-Fed Microbial (DFM) candidates that produce exogenous enzymes (protease, phytase, lipase, xylanase and cellulose) in high NSPs diets significantly reduced both viscosity and Clostridium perfringens proliferation when compared with control diets without the DFM in vitro [27,54,55]. Together, they represent a step towards the application of nutrigenomics in the context of a chicken model. The incorporation of one or more nutrigenomics techniques (in particular, assessment of the microbiome) will provide a better understanding of how dietary food components can affect physiological functions and the fundamental cellular and molecular mechanisms implicated in the digestive process of high NSPs diets in chickens.
In conclusion, rye evoked mucosal damage in turkey poults resulting in increased intestinal viscosity, increased leakage through disruption of epithelial tight junctions in the intestinal tract, and altered the microbiota composition and bone mineralization. Studies to evaluate dietary inclusion of selected DFM candidates that produce exogenous enzymes in rye fed turkey poults are currently being evaluated.
Supporting Information S1 Dataset. Dataset trial 1 and 2. (XLSX)
Author Contributions
Conceived and designed the experiments: GT JL VK. Performed the experiments: GT JL VK XH. Analyzed the data: GT. Contributed reagents/materials/analysis tools: BH. Wrote the paper: GT JD VK XH. Gave to the manuscript the format requested, made orthographic and concordance reviews, and made the changes requested by the reviewers: GT JK VK BH XH.
References
1. Salzman NH. Microbiota-immune system interaction: an uneasy alliance. Curr Opin Microbiol. 2011; 14: 99–105. doi: 10.1016/j.mib.2010.09.018 PMID: 20971034
2. Elson CO, Cong Y. Host-microbiota interactions in inflammatory bowel disease. Gut Microbes 2012; 3: 332–344. PMID: 22572873
3. Salminen S, Isolauri E. Intestinal colonization, microbiota, and probiotics. J Pediatr. 2006; 149: S115– S120.
4. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 2011; 474: 327–336. doi: 10.1038/nature10213 PMID: 21677749
5. Stepniak D, Koning F. Celiac disease—sandwiched between innate and adaptive immunity. Hum Immunol. 2006; 67: 460–468. PMID: 16728270
6. Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 1992; 102: 330–354. PMID: 1727768
7. Williamson D, Marsh MN. Celiac disease. Mol Biotechnol. 2002; 22: 293–299. PMID: 12448883
8. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009; 137: 1912–1933. doi: 10.1053/j.gastro.2009.09.008 PMID: 19766641
9. Kupfer SS, Jabri B. Celiac disease pathophysiology. Gastrointest Endosc Clin N Am. 2012; 22: 639– 660. doi: 10.1016/j.giec.2012.07.003 PMID: 23083984
10. Str?hle A, Wolters M, Hahn A. Celiac disease-the chameleon among the food intolerances. Med Monatsschr Pharm. 2013; 36: 369–380. PMID: 24266248
11. Silva MA, Jury J, Sanz Y, Wiepjes M, Huang X, Murray JA, et al. Increased bacterial translocation in gluten-sensitive mice is independent of small intestinal paracellular permeability defect. Dig Dis Sci. 2012; 57: 38–47. doi: 10.1007/s10620-011-1847-z PMID: 21822909
12. Bianchi ML. Inflammatory bowel diseases, celiac disease, and bone. Arch Biochem Biophys. 2010; 503: 54–65. doi: 10.1016/j.abb.2010.06.026 PMID: 20599670
13. Bianchi ML, Bardella MT. Bone in celiac disease. Osteoporos Int. 2008; 19: 1705–1716. doi: 10.1007/ s00198-008-0624-0 PMID: 18418638
14. James SP. Prototypic disorders of gastrointestinal mucosal immune function: Celiac disease and Crohn’s disease. J Allergy Clin Immunol. 2005; 115: 25–30. PMID: 15637543
15. Assimakopoulos SF, Papageorgiou I, Charonis A. Enterocytes’ tight junctions: From molecules to diseases. World J Gastrointest Pathophysiol. 2011; 2: 123–137. doi: 10.4291/wjgp.v2.i6.123 PMID: 22184542
16. Leffler D. Celiac disease diagnosis and management: a 46-year-old woman with anemia. JAMA 2011; 306: 1582–1592. doi: 10.1001/jama.306.14.1582 PMID: 21990301
17. Dunsford BR, Knabe D, Haensly W. Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early-weaned pig. J Anim Sci. 1989; 67: 1855–1863. PMID: 2768128
18. Hrncir T, Stepankova R, Kozakova H, Hudcovic T, Tlaskalova-Hogenova H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol. 2008; 9: 65. doi: 10.1186/1471-2172-9-65 PMID: 18990206
19. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011; 12: 5–9. doi: 10.1038/ni0111-5 PMID: 21169997
20. Campbell GL, Campbell LD, Classen HL. Utilisation of rye by chickens: Effect of microbial status, diet gamma irradiation and sodium taurocholate supplementation. Br Poult Sci. 1983; 24: 191–203. PMID: 6883150
21. Bedford MR, Schulze H. Exogenous enzymes for pigs and poultry. Nutr Res Rev. 1998; 11: 91–114. doi: 10.1079/NRR19980007 PMID: 19087461
22. Shirzadi H, Moravej H, Shivazad M. Influence of non starch polysaccharide-degrading enzymes on the meat yield and viscosity of jejunal digesta in broilers fed wheat/barley-based diet. Afr J Biotechnol. 2010; 9: 1517–1522.
23. Bedford MR, Classen HL. An in vitro assay for prediction of broiler intestinal viscosity and growth when fed rye-based diets in the presence of exogenous enzymes. Poult Sci. 1993; 72: 137–143. PMID: 8426842
24. Annett CB, Viste JR, Chirino-Trejo M, Classen HL, Middleton DM, Simko E.. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002; 31: 598–601. PMID: 12593744
25. National Research Council. Nutrient Requirements of Poultry. 9th rev. ed. Washington, DC.: National Academic Press; 1994.
26. Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012; 18: 2609–2618. doi: 10.3748/wjg.v18.i21.2609 PMID: 22690069
27. Tellez G., Latorre JD, Kuttappan VA, Kogut MH, Wolfenden A, Hernandez-Velasco X, et al. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front Gen. 2014; 5: 339. doi: 10.3389/fgene.2014.00339
28. Zhang B, Coon CN. The relationship of various tibia bone measurements in hens. Poult Sci. 1997; 76: 1698–1701. PMID: 9438284
29. AOAC International. Animal feeds. In: Horwaitz W, editor. Official Methods of Analysis of AOAC International. 17th edn., Vol. 1. Gaithersburg, MD: AOAC International; 2000. pp. 1–54.
30. SAS Institute. SAS User Guide. Version 9.1. Cary, NC.: SAS Institute Inc; 2002.
31. Murphy T, McCracken J, McCann M, George J, Bedford M. Broiler performance and in vivo viscosity as influenced by a range of xylanases, varying in ability to effect wheat in vitro viscosity. Br Poult Sci. 2009; 50: 716–724. doi: 10.1080/00071660903389950 PMID: 19946825
32. Kiarie E, Romero LF, Nyachoti CM. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. 2013; 26: 71–88. doi: 10.1017/S0954422413000048 PMID: 23639548
33. Choct M, Hughes RJ, Trimble RP, Angkanaporn K, Annison G. Non-starch polysaccharide-degrading enzymes increase the performance of broiler chickens fed wheat of low apparent metabolizable energy. J Nutr. 1995; 125: 485–492. PMID: 7876924
34. Hofacre CL. Necrotic enteritis, currently a billion dollar disease: is there anything new on the horizon. Science and Technology in the Feed Industry. Proceedings of Alltech’s 17th Annual Symposium. Lyons TP and Jacques KA, editors. Nottingham, UK: Nottingham University Press. 2001. pp. 79–86.
35. Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011; 40: 341–347. doi: 10.1080/03079457.2011.590967 PMID: 21812711
36. Bedford MR, Classen HL, Campbell GL. The effect of pelleting, salt, and pentosanase on the viscosity of intestinal contents and the performance of broilers fed rye. Poult Sci. 1991; 70: 1571–1577. PMID: 1886869
37. Teirlynck E, Bjerrum L, Eeckhaut V, Huygebaert G, Pasmans F, Haesebrouck F, et al. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Br J Nutr. 2009; 102: 1453–1461. doi: 10.1017/S0007114509990407 PMID: 19664304
38. Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005; 84: 634–643. PMID: 15844822
39. Huyghebaert G, Ducatelle R, Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 2011; 187: 182–188. doi: 10.1016/j.tvjl.2010.03.003 PMID: 20382054
40. Van Leeuwen P, Mouwen JM, Van Der Klis JD, Verstegen MW. Morphology of the small intestinal mucosal surface of broilers in relation to age, diet formulation, small intestinal microflora and performance. Br Poult Sci. 2004; 45: 41–48. PMID: 15115199
41. Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr. 2002; 41: 132–137. PMID: 12111051
42. Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci U S A. 2003; 100: 10452–10459. PMID: 12923294
43. Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004; 12: 129– 134. PMID: 15001189
44. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008; 453: 620–625. doi: 10.1038/nature07008 PMID: 18509436
45. Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012; 590: 447–458. doi: 10.1113/jphysiol.2011.219691 PMID: 22124143
46. Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PloS One 2009; 4: e6073. doi: 10.1371/journal.pone.0006073 PMID: 19562033
47. MacAuliffe T, McGinnis J. Effect of antibiotic supplements to diets containing rye on chick growth. Poult Sci. 1971; 50: 1130–1134. PMID: 5095825
48. Rennie JS, Whitehead CC, Thorp BH. The effect of dietary 1,25-dihydroxycholecalciferol in preventing tibial dyschondroplasia in broilers fed on diets imbalanced in calcium and phosphorus. Br J Nutr. 1993; 69: 809–816. PMID: 8329355
49. Capriles VD, Martini LA, Arêas JA. Metabolic osteopathy in celiac disease: importance of a gluten-free diet. Nutr Rev. 2009; 67: 599–606. doi: 10.1111/j.1753-4887.2009.00232.x PMID: 19785691
50. Kotake S, Nanke Y, Yago T, Kawamoto M, Yamanaka H. Human osteoclastogenic T cells and human osteoclastology. Arthritis Rheum. 2009; 60: 3158–3163. doi: 10.1002/art.24886 PMID: 19877050
51. Wideman RF, Prisby RD. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front Endocrinol. 2011; 3: 183–183.
52. Yegani M, Korver DR. Factors affecting intestinal health in poultry. Poult Sci. 2008; 87: 2052–2063. doi: 10.3382/ps.2008-00091 PMID: 18809868
53. Choct M. Managing gut health through nutrition. Br Poult Sci. 2009; 50: 9–15. doi: 10.1080/ 00071660802538632 PMID: 19234925
54. Latorre JD, Hernandez-Velasco X, Kogut MH, Vicente JL, Wolfenden R, Wolfenden A, et al. Role of a Bacillus subtilis direct-fed microbial on digesta viscosity, bacterial translocation and bone mineralization in neonatal poults fed with a rye-based diet. Front Vet Sci. 2014; 1: 26. doi: 10.3389/fvets.2014.00026
55. Tellez G, Latorre JD, Wolfenden R, Vicente JL, Menconi A, Wolfenden A, et al. Screening of bacteriocin-like compound synthesis (BLC) from Bacillus spp: relation of diet composition, viscosity and proliferation of Clostridium perfringens in an in vitro digestive model. Poult Sci. 2014; 93(E-suppl.1): 214.














00167-168.jpg&w=3840&q=75)