INTRODUCTION
Leucine (Leu) is a branched-chain amino acid (BCAA) that is essential for poultry species. It is one of the most potent amino acids regarding its effects on protein synthesis and degradation, energy balance regulation, modulation of insulin secretion, a nitrogen donor for muscle production and a critical regulator to initiate the translation and protein synthesis (Antony et al., 2002; Norton et al., 2006; Wu, 2009). Leu acts as a signal that regulates protein synthesis and inhibits protein degradation in various body tissues, including skeletal muscle through increasing the activity of proteins involved in mRNA translation (Rajendern et al., 2015; Wu, 2013). Moreover, it serves as a substrate for protein synthesis and increases tissue protein retention (Yin et al., 2010). Deng et al. (2013) demonstrated that a high Leu diet enhances activation of the components related to transcriptional and translational initiation in the muscle of neonatal chicks. Garlick et al. (2005) performed in vivo and in vitro studies in rats and concluded that Leu at a very high dose can stimulate muscle protein synthesis.
By considering the above-mentioned facts, it is surprising that just a few studies have been conducted on Leu requirement of modern fast-growing broilers. The reason for the lack of sufficient studies might be due to the abundance of Leu in many dietary protein sources. Leu concentration in the practical diets, based on corn-soybean meal is much higher than the previously suggested requirements; therefore, there may not be limiting amino acid in these diets. Also, the previous studies have shown that excess Leu in the purified diets will affect the requirement of the other BCAAs (valine and isoleucine) and may create a decline in feed intake, diminish the immune status and cause leg weakness and torn feathers in growing chickens (D’Mello, 1975; Farran & Tomas, 1990; Harper et al., 1984; Smith et al., 1987). However, Barbour & Lathshaw (1992) showed that the isoleucine requirement of broiler chickens in the starter period was not affected by high concentration (24.7 g/kg) of Leu in the practical diets. Waldroup et al. (2002) suggested that when the levels of valine and isoleucine are above their minimum requirements in the practical diets, it is unlikely to observe the performance reduction in broilers due to Leu excess and BCAA antagonism.
Recent evidence (Waldroup et al., 2002; Erwan et al., 2008; Erwan et al., 2009) have indicated a positive response to Leu supplementation beyond its suggested requirements by NRC (1994; 12 g/kg total Leu from 0 to 3 weeks of age), ROSS® nutrition specifications (2014; 14.4 g/kg digestible leucine for starter phase) or by Rostagno et al. (2011; 14.17 g/ kg digestible leucine for starter phase). Chang et al. (2015) observed that supplementation of Leu in neonatal broilers improves intestinal development by increasing the villus height as well as mTOR, S6K1 mRNA expressions in the jejunum and ileum. Increasing dietary Leu concentration from 13.7 to 21.7 g/kg elevated villus to crypt ratio in the duodenum, jejunum, and ileum. Erwan et al. (2008) reported that 5 g/kg Leu supplementation to a basal diet containing 17 g/kg Leu increased the carcass weight up to 9 % in growing chickens (21 to 42 days).
According to the above-mentioned studies and also due to genetic progress in the growth potential of broilers in recent decades, it seems that the Leu requirements of broiler chicks may have been increased. Therefore, the objective of this study was to evaluate the response of broilers to Leu supplementation in the practical diets and then, if there will be any response, to determine the standardized ileal digestible (SID) Leu requirement in male broiler chicks during the starter period (0 to 14 days).
MATERIALS AND METHODS
Birds and management
All experimental procedures were approved by the university animal care and use committee. A total of 280 day- old male Ross 308 broiler chicks were obtained from a commercial hatchery. They were placed in an insulated building with longitudinal sidewalls inlets on two sides of the house. The temperature was set at 32 ºC at placement and gradually declined to ensure a bird’s comfort by using a thermostatically controlled heater, exhaust fans, and cool cells. The lighting program throughout the study was 23-hour light and 1-hour dark. Feed and water were available ad-libitum to the birds.
Feed intake (FI), BWG, and FCR were measured weekly during the trial period. No mortality was observed during the experiment. On the 14th day of the experiment, two birds were taken from each replication with an average weight close to the mean weight of the pen, weighed, and processed to measure the carcass parts.
Experimental design and dietary treatments
The chicks were randomly assigned to seven levels of dietary SID Leu comprised of a basal diet (16.3 g/ kg) and six others by adding synthetic Leu in 1 g/kg increments (17.3, 18.3, 19.3, 20.3, 21.3 and 22.3 g/kg respectively) in 5 replicates of 8 birds each from 0 to 14 days of age. Diets were prepared by adding 1 to 6 g/ kg synthetic L-leucine (Ultra-pure L-leucine, Bio Basic® Canada Inc.) to the basal diet (Table 1). The basal diet used in the present study (Table 1) was formulated to meet or exceed Ross 308 recommendations (Aviagen,2014), which contained a considerable excess of Leu (16.3 g/kg dig Leu) relative to the requirement suggested by Ross 308 (14.1 g/kg). Before starting the experiment, crude protein, crude fiber, crude ash, ether extract, calcium, phosphorus, and gross energy of feeds were measured by proximate analysis methods (AOAC, 1990). Amino acids content in corn and soybean meal were determined by high-performance liquid chromatography (Knauer, Germany) following hydrolysis by hydrochloric acid (6N) and derivatization by orthophthalaldehyde (OPA). SID values for all amino acids were calculated using the AminoDat5® software (Evonik®, Germany).
Statistical analysis
The experimental design was a completely randomized design with 7 treatments and 5 replicates. The data were subjected to analysis of variance (ANOVA) by the general linear model procedure of SAS software (SAS Institute, 2003). Tukey’s HSD test was used to determine significant differences among treatment means. Contrast statements were used to determine the linear and quadratic trends. The significance level was considered p< 0.05 and the tendency was considered p< 0.1. The SID Leu requirements were estimated using Linear broken-line (LBL), quadratic broken-line (QBL), and quadratic polynomial (QP) regression models (Robbins et al., 2006). The best model was selected based on goodness of fit criteria such as coefficient of determination (R2) and residual sum of square (SSE).
Table 1 – Composition and nutrients levels of the basal diet (g/kg).
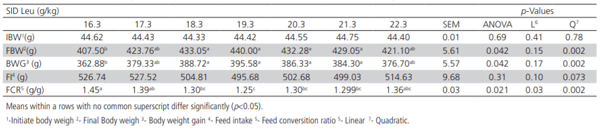
Table 2 – Effects of dietary Leu on performance of male broilers from 0 to 14 days.
RESULTS
The effects of SID Leu levels on final body weight (FBW), BWG, FI, and FCR from 0 to 14 days of age are presented in table 2. There were significant effects (p< 0.05) of Leu supplementation on FBW, BWG, and FCR. The FBW of all broilers fed 18.3 to 21.3 g/kg SID Leu diets was higher than that of the basal diet (ANOVA p=0.042). The BWG was increased quadratically (p=0.002) by increasing the levels of SID Leu, being highest in 19.3 g/kg SID Leu group. FI was not affected by dietary SID Leu levels. FCR was significantly improved (ANOVA p< 0.05) in broilers fed 18.3 to 21.3 g/kg SID Leu relative to the basal diet. FCR decreased quadratically (p=0.0017) by increasing SID Leu levels, and the best FCR was achieved by broilers fed 19.3 g/kg, SID Leu.
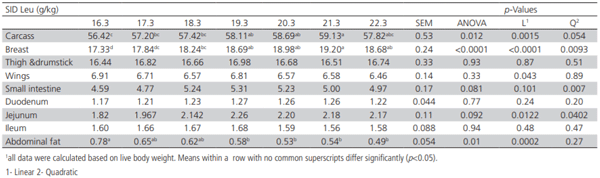
The effects of Leu supplementation on carcass, breast, thigh and drumstick, wings, small intestine, duodenum, jejunum, ileum, and abdominal fat percentages of broilers at 14 days are presented in Table 3. Carcass (p< 0.012), breast (p< 0.0001), and abdominal fat (p< 0.01) percentages are affected significantly by different levels of SID Leu. Broiler chicks fed diets containing 19.3 to 21.3 g/kg of SID Leu had higher carcass yield compared to the diet containing 16.3 g/kg of SID Leu (basal diet). Broilers fed diets containing 18.3 to 22.3 g/kg
SID Leu had higher breast percentages relative to the basal diet (p< 0.0001) being highest in 21.3 g/ kg SID Leu. The abdominal fat percentage decreased linearly (p=0.0002) as dietary SID Leu increased up to 22.3 g/kg. The SID Leu levels did not affect thigh and drumstick, and wings percentages. The effects of SID Leu levels on the small intestine and jejunum percentages tended to be significant (p=0.081 and p=0.09, respectively).
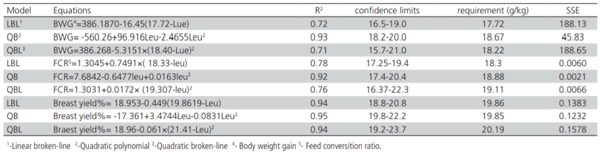
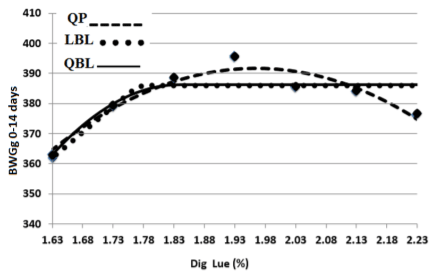
The optimum levels of SID Leu for BWG of broiler chicks in the starter phase (0 to 14 days) were determined by a quadratic polynomial (QP), quadratic broken-line (QBL), and Linear broken-line (LBL) models (Table 4 and Figure 1). The estimated dietary SID Leu requirement by QP model based on BWG response was 18.67 g/ kg at 95% of the maximum response. Using the LBL model, the SID Leu requirement was estimated at 17.72 g/ kg with a response plateau for BWG at 386.187 grams. Using the QBL model, the SID Leu requirement of 18.22 g/ kg at 99% of the maximum response was estimated. Based on the goodness of fit criteria, the best fit was achieved by the QP model and the estimated requirement for BWG was 18.67 g/ kg, SID Leu.
Table 3 – Effect of Leu supplementation on carcass and visceral composition (%) of broilers1 (14 days).
Table 4 – Estimation of digestible Leu requirement (g/kg) of broiler chicks in the starter period.
Figure 1 – The Optimum SID Leu for BWG(g) of starting male broilers determined by Quadratic polynomial (QP), Quadratic broken-line (QBL) and linear broken-line (LBL) models. The equations are presented in table 4.
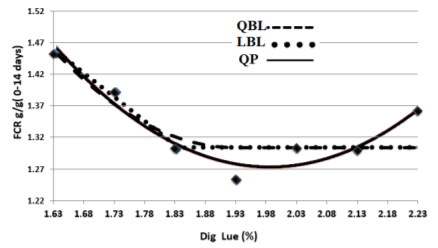
Figure 2 – The Optimum SID Leu for the FCR(g/g) response of starting male broilers determined by Quadratic polynomial (QP), Quadratic broken-line (QBL) and linear broken-line (LBL) models. The equations are presented in table 4.
The SID Leu requirements for minimum FCR obtained by LBL, QP, and QBL models were 18.22, 18.88, and 19.11 g/kg, respectively (Figure 2, Table 4). Considering FCR as a response, the best fit was attributed to the QP model, and the SID Leu requirement was estimated to be 18.8 g/kg.
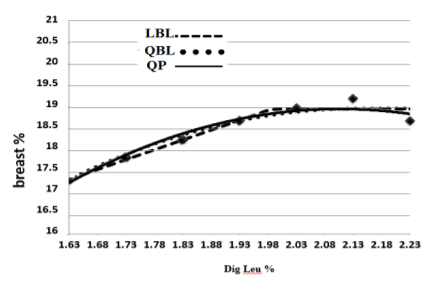
The SID Leu requirements for maximum breast yield were obtained 19.86, 19.85, and 20.19 g/kg by LBL, QP, and QBL models, respectively (Figure 3, Table 4). The best fit was attributed to the QP model, and therefore the estimated dietary requirement based on breast yield was 19.85 g/ kg, SID Leu.
Figure 3 – The Optimum SID Leu for breast yield of starting male broilers determined by Quadratic polynomial (QP), Quadratic broken-line (QBL) and linear broken-line (LBL) models. The equations are presented in table 4.
DISCUSSION
The results of the current study clearly showed that dietary Leu supplementation improved BWG, FCR, carcass, and breast yield and decreased abdominal fat percentage of broilers. These results are consistent with the recent studies that showed that moderate excess of Leu improved performance parameters and carcass characteristics. Chang et al. (2015) fed diets containing 13.7, 17.7, 21.7, and 25.7 g/kg of Leu to broilers and achieved better average daily gain and gain to feed ratio in broilers fed the diet with 21.7 g/ kg Leu compared with the 13.7 g/kg of Leu. Erwan et al. (2008) reported a 9% improvement in carcass weight of broilers by supplementation of 5 g/kg L-leu (dietary Leu 21.6 g/kg), which is in agreement with our findings. Erwan et al. (2009) found that FI, BWG, and FCR were not affected by the diet containing 20.6 g/ kg Leu; however, the performance decreased when dietary Leu concentration increased to 23.4 g/kg. They reported that supplementation of 5 g/kg L-Leu increased breast meat yield by up to 8.5%.
In this study, the effects of the Leu addition to the basal diet tended to be significant on relative weights of small intestine and jejunum. It seems that a part of the improvement in BWG, FCR, carcass, and breast yield may be attributed to the role of Leu in maintaining gut health. Chen et al. (2016) showed that graded digestible Leu levels of 11.6, 19.4, and 27.3 g/kg improved BWG, FI, FCR, and breast muscle weight and tended to increase villus height. They attributed the improvement in FCR to the increased villus height in 19.6 and 27.3 g/kg Leu diets. Chang et al. (2015) fed diets with 13.7, 17.7, 21.7, and 25.7 g/kg of Leu to broilers and showed that villus height in the jejunum and ileum increased along with the Leu levels from 13.7 to 21.7 g/kg. They showed that villus height of jejunum was significantly higher in 21.7 g/ kg leu than that in 13.7 g/kg on days 7 and 14 and the ratio of villus height to crypt depth (V:C) in the duodenum, jejunum, and ileum increased significantly on day 21. It has been determined that Leu acts as a regulator to promote intestinal development, nutrient transporters, and immune-related function and then improve gut health (Ren et al., 2016). Leu supplementation can maintain intestinal health by enhancing tight junction (Jiang et al., 2015) and also improves the proliferation of intestinal epithelial cells, villus height; however, intestinal growth inhibited when the level of Leu increased to 25.7 g/kg (Ren et al., 2015). The expression of the mammalian target of rapamycin (mTOR) and S6K1 transcription factors in jejunum and ileum increased with increasing Leu levels from 13.7 to 21.7 g/kg; which indicating that Leu promotes intestinal development through activation of mTOR and its downstream pathways (Chang et al., 2015).
Earlier studies, however, reported a reduction of broiler performance by dietary Leu supplementation (D’Mello et al.,1970b; D’Mello et al.,1970a; Edmonds et al.,1987; Farrant et al.,2003). In the present study, the adverse effect of Leu addition was only slightly observed by 6 g/kg Leu supplementation to the basal diet (22.3 g/kg SID Leu). Moreover, Leu supplementation had no significant effect on feed intake. Therefore it can be concluded that classical BCAA antagonism (Edmonds et al., 1987; Farrant et al., 2003) has not occurred. On the other hand, Leu addition stimulates protein synthesis and inhibits protein degradation. This phenomenon is known as the Leu paradox (Vinnna et al., 2010). Our findings also confirm the opinion of Waldroup et al. (2002) and Barbour & Latshaw (1992), that in practical diets, when levels of Val and Ile are above their minimum requirements, it is unlikely that BCAA antagonism due to Leu excess occurs. The adverse effects of excess Leu have been demonstrated mainly in diets containing marginal or deficient levels of Val and Ile (D’Mello et al.,1970a; D’Mello et al., 1970b; Harper, 1984), in low-protein diets (Farrant et al, 2003; Spina- Rojas et al., 2016), purified or semi-purified diets in which synthetic isoleucine and valine have been used to meet the requirements of these two amino acids (Harper et al.,1984; D’Mello, 2003; Spina- Rojas, 2016; Zeitz et al, 2019). Thus stimulation of protein synthesis by Leu depends on the availability of all essential and non-essential amino acids (Wilson et al. 2011); so in diets with sufficient levels of all amino acids, the antagonism between BCAAs and a moderate level of Leu supplementation does not occur. This conclusion is in agreement with Sedghi et al. (2015) that dietary excess of Leu is well tolerated when diets contain sufficient levels of Ile and Val.
The positive effects of Leu supplementation on carcass and breast yields in this study may be due to stimulating protein synthesis, particularly the activity of proteins involved in the translation process, which is critical for cells to control protein synthesis. Leu has been shown to stimulate protein synthesis in skeletal muscle, and also inhibit protein degradation (Anthony et al., 2002; Baker, 2012). The anabolic mechanism of Leu is performed by activation of mRNA translational machinery through the mTOR in an insulin-dependent or independent manner (Baker, 2012; Zhang et al., 2017). Szczeniak et al. (2015) demonstrated that Leu or its metabolites, i.e., α-isocaproate and β-hydroxy-β-methyl butyrate strongly increase muscle protein synthesis in neonatal pigs. They indicated that the stimulation of protein synthesis by Leu is dependent on the availability of other amino acids; Leu supplementation in protein and energy-restricted diets only enhanced mTOR pathway activation without increasing muscle protein synthesis in neonatal pigs. A recent study by Deng et al. (2013) showed that 17.3 and 20.3 g/kg of dietary Leu could stimulate the mTOR pathway in neonatal chicks compared to those fed 14.3 g/kg Leu. They concluded that high dietary Leu level in neonatal broiler chicks, increases mTOR, S6K1, and 4E-BP1 mRNA expression within pectoral muscles.
In the present study, the abdominal fat percentage decreased linearly with increasing dietary Leu levels and lowest abdominal fat was observed by broilers fed 22.3 g/kg SID Leu diet. Leu shifts energy partitioning from adipocytes to muscle cells, resulting in decreased energy storage in adipocytes and increased fatty acid utilization in muscle cells (Li et al., 2011). The other reason for reducing adipose tissue can be attributed to the synergistic effect between Leu and leptin (Zhang et al., 2017). Ospina-Rojas et al. (2016) showed that increasing levels of dietary Leu (10, 12, 14, 16, or 18 g/kg) linearly reduced serum concentrations of triglycerides and β-hydroxybutyrate in male Cobb-500 broilers from days 8 to 17 of age. They concluded that low-protein diets increase fat content in poultry carcass, and valine and Leu supplementation can improve carcass quality by reducing fat deposition and increasing retention of lean tissue.
The SID Leu requirements were estimated to be 18.67, 18.88, and 19.85 g/kg for BWG, FCR, and breast meat yield, respectively. The SID Leu requirement determined in this study (mean=19.13 g/kg and Leu: Lys ratio=1.594, Table 4) was higher than that of NRC (1994), Farran & Thomas (1990), Baker (2012), Rostagno et al. (2011) and Sedghi et al. (2015). The difference may be due to levels of other BCAAs in the basal diet, type of the basal diet (purified, semi-purified or practical), the genetic potential of modern broilers, and the statistical methods. Sedghi et al. (2015) estimated dietary SID Leu requirements ranging from 13.45 to 16 g/kg by using different statistical methods. Moreover, the highest BWG and lowest FCR were obtained by dietary SID Leu level of 17.48 g/kg in their field experiment, while researchers did not comment on that. Barbour & Latshaw (1992) indicated that chicks fed purified diets gained more slowly and had a lower requirement for nutrients. The basal diet used in the present study was a practical high protein diet that did not limit growth potential and not elicit BCAA antagonism.
The quadratic polynomial model (QP) fitted the response data better than other statistical models (LBL and QBL). The quadratic polynomial trend of Leu levels on response criteria was also reported by Franco et al. (2017). Many statistical models have been developed to determine the requirements of amino acids, but the choice of appropriate model will depend on the best fit of data (Franco et al., 2017). Baker et al. (2003) suggested that the linear broken-line method is the best way to estimate the amino acid requirement because it estimates the maximum response with a minimum amount of amino acid regardless of economic considerations and safety margins. However, one of the conditions when using the linear broken-line model is the plateau of response after the break-point; if there is a decrease of performance beyond the break-point, the quadratic polynomial model will fit the data better and clearly describe nutrient toxicity (Aude et al., 2002).
In conclusion, Leu supplementation improved performance and carcass traits of starting broiler chicks fed with a practical high protein diet. The SID Leu level of 19.13 g/kg (Leu to Lys ratio of 1.594) was recommended for broilers during the starter phase according to the observed responses to Leu supplementation in the present experiment. Due to the unique role of leucine in the stimulation of protein synthesis and broiler growth, further research is warranted in this field.
This article was originally published in Revista Brasileira de Ciência Avícola, 2020 / v.23 / n.1 / 001-008. http://dx.doi.org/10.1590/1806-9061-2019-1176. This is an Open Access article distributed under a Creative Commons Attribution License. 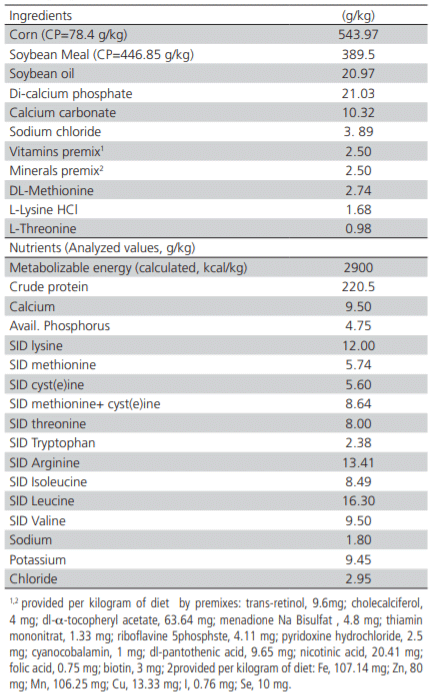









.jpg&w=3840&q=75)