Phytase and phosphorus in Poultry Nutrition
Phytase and Phosphorus: An overview of the U.S. Poultry Industry
Approximately 70% of the cost of producing a kilogram of chicken, a kilogram of turkey or a dozen eggs is attributed to feed. Therefore, any improvements in feed cost, while maintaining performance and production goals are extremely beneficial. Energy, protein, and phosphorus, in order, are the three most expensive nutrients in producing poultry meat and eggs - energy and protein required to deposit lean tissue, and phosphorus required to support the skeletal and egg structures. Clearly, the phosphorus nutriture of poultry nutrition is an important economic, and environmental, issue.
The expense of P results from the low content and low biological availability (bioavailability) of P contained in the commonly-used grains and oilseed meals (Table 1). The low content of bioavailable P in feedstuffs, combined with the birds' requirements for P that exceed that provided by the grains and oilseed meals drives the need for inorganic P supplementation, from sources such as mono- or dicalcium phosphate. Although phosphate prices have fluctuated over the past few years, they have recently stabilized, and the poultry industry dominates the market share. Phosphorus supplementation accounts for $5 to 6 per ton in most broiler and laying hen rations. Biehl et al. (1995) reported that almost $1 billion is spent annually to supplement animal diets worldwide with P. The low bioavailability of P in grains and oilseeds also leads to large amounts of consumed P being excreted. The excreta is applied to soil, from which P is at risk of washing into ground water, contaminating ponds, streams, lakes, rivers and oceans. The excess P stimulates algae growth, which depletes marine life of oxygen, leading to morbidity and mortality of fish present in these habitats (Ryden et al., 1973). Strict legislation in Europe controls the amount of P that can be applied to the soil. The United States is facing similar regulations, as in the case of the Delmarva Peninsula (Marbery, 1998; Moore et al., 1999) which constitutes areas of Delaware, Maryland and Virginia along the Atlantic Coastal Plain. The Delmarva Peninsula is a large broiler-producing area with excessive poultry waste to dispose of on a relatively small amount of land.
The issues of cost effectiveness and excess P in poultry production can be addressed on several fronts: the P requirements of birds at different growth stages, the ingredients available to meet that requirement (and their cost), and the bioavailability of P associated with each of those ingredients. Knowledge of the birds' bioavailable P requirement is imperative. In general, the poultry industry utilizes a significant safety margin for available P, which results in increased feed cost and excessive excreta P. Cobb Broiler Nutrition Supplement (2004) recommends 0.45% available P during the starter phase 0-3 weeks of age. It is important to note the difference between recommendation and requirement. Recommendations are typically set and based upon requirements established under scientific parameters. Requirements are also impacted by the criterion with which they are quantified, e.g., maximum growth performance, maximum bone mineralization or strength. Work at the University of Arkansas (Waldroup et al., 2000) demonstrated that the minimal requirement for non-phytate P (nPP) in broiler chicks 0-3 weeks of age was 0.39% based on tibia ash responses and 0.32% based on body weight responses. Further work by Lee et al. (2006) at Texas A & M validated 0.30-0.35% aP supported optimal tibia ash and body weight gain in chicks 0-21 d of age. The nPP requirement for 3-6 week broilers was estimated to be 0.33% for optimal bone ash concentration (Waldroup et al., 2001). Non-phytate P levels were found to be 0.31, 0.23 and 0.22% at 49, 56 and 63 days, respectively, to maximize bone (tibia) ash weight (Yan et al., 2003).
Manangi and Coon (2008) suggested broilers have a physiological threshold for P, intake above which P would no longer be absorbed. In the presence of 0.50% Ca, the threshold was 0.23% aP; at 0.90% Ca, the threshold was 0.26-0.28% aP. Beyond the threshold, excess P was not absorbed and an increase in excreta total P, plasma iP, and total urine P was observed. In all studies assessing aP requirements, excess inorganic P does not improve egg yield, meat yield, body weight, or feed conversion (Boling et al., 2000; Yan et al., 2003). However, one must instill a "safety margin" to account for ingredient variability, variations in feed intake, etc. As demonstrated in the aforementioned studies, the aP requirement for broilers 0-21 d was estimated to be between 0.30-0.35%; the industry feeds an average of 0.45% aP during the starter phase.
In order to meet the birds' requirement for P with a minimal amount of margin, knowledge of the content and bioavailability of the available feedstuffs is important. The range in both content and bioavailability of P from a wide range of feedstuffs utilized in poultry production was previously shown in Table 1. Grains provide a great deal of energy, but are a poor source of available P, since most (60-80%) of the P is stored in a form called phytic acid (or phytate), from which P is completely unavailable by itself. Rendered animal by-products (meat and bone meal) are decent sources of available P, as well as good sources of energy and protein. Then obviously inorganic phosphates such as monocalcium phosphate or defluorinated phosphate are concentrated and highly available sources of P. An effective and now widely-accepted ingredient that can be used to meet the P requirement of birds is dietary phytase, a feed-additive enzyme that increases the availability of phytate-bound P in grains and oilseed meals. The action of phytase can have a two-fold effect: the improved utilization of phytic acid-bound P reduces the need for inorganic P-supplementation, while concomitantly reducing the potential environmental impact of animal production through reduced P excretion. Hence, the ability to reduce or eliminate the need for inorganic phosphate supplementation through maximum utilization of phytase can have both economically- and environmentally-beneficial effects in poultry production.
Phytase feed additives have been available commercially since the mid-1990's, and there are currently five commercially-available products for use in feeding poultry in the U.S. These enzymes have been produced from fungal organisms and bacterial organisms, and produced through fungal and yeast organisms. Laboratory in vitro work has quantified differences among the phytases in pH stability, digestive enzyme resistance, binding of the phytate molecule, and the speed of its action (Igbasan et al., 2000; Rodriguez et al., 1999a, b). These characteristics are important as this enzyme needs to be active in the proventriculus/gizzard area of the digestive tract, a highly proteolytic and acidic environment (Tamim et al., 2004; Yu et al., 2002) where the digesta resides for only a short period of time prior to moving into the upper small intestine. The small intestine environment is similarly highly proteolytic, but more basic (approximately 6 pH units) in which several minerals can bind to the phytate and render it unavailable for phytase to bind, reducing its efficacy. Ultimately, however, the effectiveness in the animal is what is of primary importance in determining the value of a phytase.
The degree of processing which complete diets undergo is a key factor in the feed-mill application of phytase technology. Phytase enzymes are proteins that are susceptible to destruction induced by heat, moisture, and pressure, all characteristics of feed processing techniques such as pelleting or expanding. Dry forms of phytase are significantly degraded when feed is processed in this type of technique. For this reason, this form of phytase is generally only used in situations where feed is not put through a pelleting or expanding process. Coating technologies to confer heat and moisture stability to that dry product have allowed dry products to be used in pelleting applications. Liquid forms of phytase that can be applied after the pelleting process allow the enzyme to bypass the processing step. In the U.S., an estimated 30% of the broiler industry utilizes a liquid form of phytase, applied post-pelleting.
Meeting available P requirements with available ingredients of known P value with minimal margin and least cost is the practical goal of poultry production systems. The economic value of phytase is dependent upon the amount of P that it can replace and the cost associated with supplementing P through the phytase vs. other available ingredients. Firstly, however, the quantitative estimates of P-release determined for phytase in young chickens and turkeys must be verified and have supported full growth and bone mineralization in qualitative, long-term feeding studies. Pillai et al. (2006) reported than replacing 0.10% up to 0.20% P with 300 and 1,000 FTU/kg phytase (E. coli-derived product), respectively, in broilers yielded no differences in final body weights (2.8 kg), bone ash (%), gain/feed ratio, or carcass yield at 7 wk of age (Table 2). An additional experiment with commercial, floor-pen-reared broiler chickens carried out to 8 wk of age revealed no differences in weight gain, gain/feed ratio, bone ash (%), or incidence of broken bones in response to replacement of 0.20% P by 1,000 FTU/kg of the same E. coli-derived phytase (Table 2; JBS United, Inc., unpublished data). Replacement of 0.20% P by 1,000 FTU/kg E. coli-derived phytase in turkey toms fed pelleted corn-soybean meal diets maintained body weight, feed efficiency, breast yield, and bone mineralization compared to a P-adequate control diet over a 19-wk grow-out period (Table 3). Work in second-cycle laying hens showed that 150 FTU/kg E. coli-derived phytase added to a corn-soybean meal diet devoid of inorganic P (0.10% estimated available P) maximized egg production, egg weight, and feed intake relative to a P-supplemented positive control (Augspurger et al., 2007).
Practical formulation seeks to meet the determined nutrient targets with a minimal amount of margin for the lowest cost. Feed ingredient costs, animal nutrient requirements and performance, feed milling considerations, and governmental regulations, among probably a host of other issues, converge together to determine the most profitable means of providing the needed nutrients to the animals. The replacement of 0.10 or 0.16% P by phytase in broiler diets reduced the inclusion rate of defluorinated phosphate by 0.5 to 0.8%, reducing the unavailable P from 0.3% in the non-phytase diet to 0.14% in the diet with the highest inclusion of phytase. The use of phytase in these scenarios could ultimately effect a reduction in P-excretion of up to 50%.
Poultry production in the U.S. operates in an economic environment of tight profit margins, which elevates the value of technologies and processes that can reduce cost whilst maintaining productive performance of the flocks. Practical knowledge of the performance and nutritional requirements of the birds is vital, allowing systems to confidently feed for maximum profitability with best-cost practices that can minimize the impact poultry production can have on the environment.
Literature cited
Applegate, T.J., D.M. Webel and X.G. Lei. 2003. Efficacy of an E. coli-derived phytase expressed in yeast on phosphorus utilization and bone mineralization in turkey poults. Poult. Sci. 82:1726-1732.
Augspurger, N.R., D.M. Webel, X.G. Lei, and D.H. Baker. 2003. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci. 81:474-483.
Augspurger, N.R., D.M. Webel, X.G. Lei, and D.H. Baker. 2007. An E. coli phytase expressed in yeast effectively replaces inorganic phosphorus for finishing pigs and laying hens. J. Anim. Sci. 85:1192-1198.
Biehl, R.R., D.H. Baker, and H.F. DeLuca. 1995. 1α-hydroxylated cholecalciferol compounds act additively with microbial phytase to improve phosphorus, zinc and manganese utilization in chicks fed soy-based diets. J. Nutr. 125:2407-2416.
Boling, S.D., M.W. Douglas, M.L. Johnson, X. Wang, C.M. Parsons, K.W. Koelkebeck, and R.A. Zimmerman. 2000. The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens. Poult. Sci. 79:224-230.
Igbasan, F.A., K. Manner, G. Miksch, R. Borriss, A. Farouk, and O. Simon. 2000. Comparative studies on the in vitro properties of phytases from various microbial origins. Arch. Anim. Nutr. 53:353-373.
Kornegay, E.T. 2001. Enzymes in Farm Animal Nutrition. Eds. M.R. Bedford and G.G. Partridge. pp.237-271.
Managi, M.K., and C.N. Coon. 2008. Phytate phosphorus hydrolysis in broilers in response to dietary phytase, calcium, and phosphorus concentrations. Poult. Sci. 87:1577-1586.
Marbery, S. 1998. Maryland first state to regulate phosphorous. Feedstuffs. Vol. 70 no. 17 pp.1,4.
Moore, P. A., Jr., T. C. Daniel and D. R. Edwards, 1999. Reducing phosphorus runoff and improving poultry production with alum. Poultry Sci. 78:692-698.
National Research Council. 1994. Nutrient Requirements of Poultry. 9th Ed.
Pillai, P.B., T. O'Connor-Dennie, J.L. Emmert. 2006. Efficacy of an Escherichia coli phytase in broilers fed adequate or reduced-phosphorus diets and its effect on carcass characteristics. Poult. Sci. 85:1737-1745.
Poultry USA. 2010. Watt Publishing. February issue.
Rodriguez, E., Y. Han, and X.G. Lei. 1999a. Cloning, sequencing, and expression of an Escherichia coli acid phosphatase/phytase gene (appA2) isolated from pig colon. Bioch. Biophys. Res. Comm. 257:117-123.
Rodriguez, E., J.M. Porres, Y. Han, and X.G. Lei. 1999b. Different sensitivity of recombinant
Aspergillus niger phytase (r-PhyA) and Escherichia coli pH 2.5 acid phosphatase (r-AppA) to trypsin and pepsin in vitro. Arch. Bioch. Biophys. 365:262-267.
Ryden, J.C., J.K. Syers, and R.F. Harris. 1973. Phosphorus in runoff and streams. Pages 1-44 in: Advances in Agronomy. Vol. 25. Academy Press, New York, NY.
Tamim, N.M., R. Angel, and M. Christman. 2004. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 83:1358-1367.
Waldroup P.W., J.H. Kersey, E.A. Saleh, C.A. Fritts, F. Yan, H.L. Stilborn, R.C. Crum Jr., and V. Raboy. 2000. Nonphytate phosphorus requirement and phosphorus excretion of broiler chicks fed diets composed of normal or high available phosphate corn with and without microbial phytase. Poult Sci. 79:1451-1459.
Yan, F., J.H. Kersey, C.A. Fritts, and P.W. Waldroup. 2003. Phosphorus requirements of broiler chicks six to nine weeks of age as influenced by phytase supplementation. Poult. Sci. 82:294-300.
Yu, B., T.T.T. Lee, and P.W.-S. Chiou. 2002. Effects of sources of protein and enzyme supplementation on protein digestibility and chime characteristics in broilers. Br. Poult. Sci. 43:424-431.
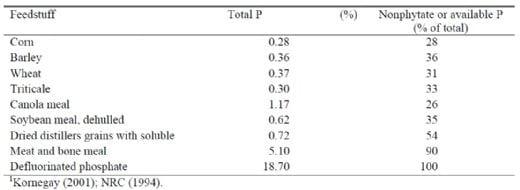
Table 2. Utilization of quantitative efficacy estimates of an E. coli-derived phytase (ECP) maintained growth performance and bone mineralization relative to control diets containing only inorganic phosphorus to meet dietary available phosphorus requirements1.
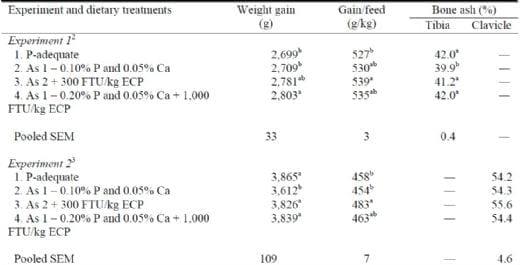
2Growth values are means of 7 pens of 20 male chicks, while bone data are means of 7 pens of 5 randomly selected birds per pen. Chickens were fed starter diets (0.48% aP in P-adequate diet) from 0 to 18 d, grower diets (0.42% P) from 18 to 32 d, finisher diets (0.38% aP) from 32 to 40 d, and withdrawal diets (0.34% aP) from 40 to 50 d posthatch.
3Values are means of 4 pens of 15 male chicks. Chickens were fed starter diets (0.43% aP in P-adequate diet) from 0 to 18 d, grower diets (0.40% P) from 18 to 32 d, finisher diets (0.37% aP) from 32 to 40 d, and withdrawal diets (0.35% aP) from 40 to 56 d posthatch.
abMeans with different superscripts are different, P < 0.05.

Very informative, phytase is prooven to be very in the broiler chicken industry

Dear Sir,
We are very much thankful for providing us and updating us on the OPTIMUM Nutrition in terms of Phosporus utility in poultry ration.
Your study is very elaborative but it will be complete if you will update us on the "impact of different water qualities on the efficay of Phytases"
If you have a data or case study material related with below topics/parameters please update us.
1) Any differences in the efficay of Phytases as per Geographical conditions viz. Asia, Australia, Europe etc?
2) Is water quality Viz. heavy metals, hardness, PH etc of water can change the efficay of Enzymes?
3) You have stated that "phytase derived from an E. coli strain released 0.10 to 0.20% P from corn-soybean meal diets at activity concentrations of 250 to 1,000 phytase units (FTU) per kilogram of complete feed (Applegate et al., 2003; Augspurger et al., 2003; 2004; 2007; Pillai et al., 2006)." Is this applicable in Indian Continent too?
Awaiting your feedback.
Thanks and regards,
Dr Jaydip













.jpg&w=3840&q=75)