phosphorus and nitrogen excretion by caged broilers
Allzyme Phytase reduces phosphorus and nitrogen excretion by caged broilers and by broilers in conventional housing
Published: December 20, 2007
By: F. W. EDENS, C. R. PARKHURST and G. B. HAVENSTEIN (Courtesy of Alltech Inc.)
In 1997, environmentalists’ efforts to control water pollution due to run-off from poultry and livestock farms resulted in legislation being introduced into the 105th US Congress (SB 1323- Animal Agriculture Reform Act and HR 3232- Farm Sustainability and Animal Feedlot Enforcement Act) that would have set national standards on pollution from poultry and livestock farms. These bills did not become law, but new legislative initiatives undoubtedly will be submitted in the 106th Congress and at the state level.
Similar legislation was proposed and passed by the Maryland General Assembly (Water Quality Improvement Act of 1998 and the Nutrient Management Practices Act of 1998) mandating that by the end of the year 2000, all feeds for monogastric animals must be supplemented with a phytase enzyme or other additives that reduce phosphorus in poultry and livestock wastes to the maximum extent that is commercially and biologically feasible. In 1995 there was a legislative mandate in The Netherlands that called for a 30% reduction in phosphorus content in manure. This goal was partially attained in the poultry sector by reducing dietary inorganic phosphorus and supplementing the diets with a microbial phytase (Simons et al., 1990; 1992; Van der Klis and Versteegh, 1994).
INCREASED PHYTATE PHOSPHORUS AVAILABILITY WITH DIETARY PHYTASE SUPPLEMENTATION
Dietary supplementation of phytase enzymes can affect the concentration of phosphorus in poultry and livestock wastes via its ability to liberate phytate phosphorus contained in the cell walls of feed grains.However, this liberation of phytate phosphorus can only be accomplished if a concomitant reduction is made in supplemented dietary inorganic phosphorus and calcium.
Phytate forms acid salts with mineral cations such as calcium, magnesium, copper, zinc, iron and potassium thereby reducing mineral solubility and availability (Erdman, 1979; Kornegay, 1996a,b). When acted upon by phytase enzyme, these cations are released much like phosphorus. Consequently, increased availability of these minerals will result in increased retention by chickens given phytase (Sebastian et al., 1996a,b). Aoyagi and Baker (1995) demonstrated reduced copper utilization in chickens fed phytase supplemented diets. The reduced copper utilization by phytase treated chicks may have been the result of increased zinc release from the phytate (Roberson and Edwards, 1994). Yi et al. (1996a,b) reported that phytase improved zinc utilization and retention in broilers fed a low zinc corn-soybean protein isolate diet.
Furthermore, Biehl et al. (1995) reported that manganese availability was increased in broiler diets supplemented with phytase. Therefore, it appears that phytase incorporation into the diets of chickens can effectively increase retention of certain minerals provided the supplementation of those minerals to the diet is reduced, and this should effectively reduce the level of minerals such as copper, zinc, and phosphorus that can act as water and soil pollutants from land applied poultry litter. Thus, phytase incorporation in poultry and livestock diets makes organic phosphorus in feed grains more available and reduces the amount of inorganic phosphorus thatmust be routinely added to diets. This nutrient management scheme would decrease the amount of phosphorus in manure and ultimately there would be a reduction in the leaching of this nutrient into ground and surface waters near poultry and animal farms.
Approximately 67% of the phosphorus in plant tissue is in the form of phytate phosphorus (myoinositol hexakisphosphate) (Cromwell, 1980; Heinzl, 1996) which is only minimally available to monogastric animals since they lack the phytase enzyme that hydrolyzes phytic acid to inositol and orthophosphate (Peeler, 1972).
Supplementation of phytase enzyme in the primarily corn-soybean meal diet of broiler chickens improves the availability of phytate-bound phosphorus (Nelson et al., 1971; Simons et al., 1990; 1992; Sauveur, 1993; Roberson and Edwards, 1994; Denbow et al., 1995). Phytate phosphorus content of corn is 68% of the total phosphorus, and in soybean meal phytate phosphorus represents 60% of the total phosphorus (Stillborn, 1998). Simons et al. (1990) demonstrated that in three week old broilers the availability of dietary phosphorus could be increased up to 65% by means of supplemental dietary phytase while reducing fecal phosphorus by 50%. According to Williams (1997), inclusion of phytase enzyme into the diets of broilers contributes to increased energy and protein density and permits the usage of cheaper feed ingredients. Furthermore, inclusion of feed grains with phytase activity such as wheat, triticale, rye, or their by-products resulted in better phosphorus utilization (Pointillart et al., 1993).
POULTRY FECAL PHOSPHORUS AND NITROGEN
Poultry manure, due to higher dry matter content and relatively high amounts of uric acid and other ureides, can be very high in phosphorus and nitrogen in comparison to pig feces, but poultry manure contains less phosphorus and nitrogen than ruminant feces (Stillborn, 1998). Uric acid and other ureides are forms of urea and can be toxic to crops. Schmitt and Rehm (1998) indicated that dry matter content of poultry manure ranged from 22 to 29% (without and with bedding, respectively), total nitrogen from 27 to 20 lbs/ton (without and with bedding, respectively), phosphorus from 20 to 16 lbs/ton (without and with bedding respectively), and potassium from 17 to 13 lbs/ton (without and with bedding, respectively).
Therefore, when farmers apply poultry litter to meet plant nitrogen needs, phosphorus often exceeds plant needs. Repeated application of manure on the same fields can result in a significant build-up of soil phosphorus, zinc, and copper which might then become a potential source of surface water contamination as run-off. As a result of Vice President Gore’s policy address in October of 1997, in the United States future land application of animal wastes may no longer be based simply on nitrogen content of the wastes, but rather phosphorus may become the limiting factor. Using phosphorus as the limiting plant nutrient for field application of poultry and animal wastes, farmers will be limited to application of 25 to 50% as much manure or be forced to spread manure on fields only one time every three to four years.
COMPOSTING INFLUENCE ON RELATIVE CONCENTRATIONS OF PHOSPHORUS AND NITROGEN IN POULTRY MANURE
Composting of poultry litter results in significant losses of nitrogen by ammonia volatilization. Schmitt and Rehm (1998) reported 15 to 35% nitrogen loss of daily scrape and haul manure, 20 to 40% loss of nitrogen in manure pack, and a 40 to 60% nitrogen loss when manure was composted in open lot. Thus, the amount of phosphorus in manure relative to nitrogen content can increase significantly depending upon the way poultry manure is handled. The lower the nitrogen concentration in manure, the more phosphorus becomes a limiting factor to land application rates if agronomic nutrient application rates are maintained.
PHYTASE INFLUENCE ON PHOSPHORUS AND NITROGEN CONCENTRATIONS IN POULTRY MANURE
A significant reduction in poultry manure phosphorus can be achieved via the use of microbial phytase in feed. This can reduce the nitrogen:phosphorus ratio in poultry wastes. Balander and Flegal (1997) studied the effect of feed supplemented with Allzyme Phytase in layer diets and reported a 16% reduction in fecal phosphorus from laying hens fed inorganic phosphorus at 80% of NRC requirements and a 25% reduction in fecal phosphorus from laying hens fed 60% of NRC requirements. A 38% decrease in fecal phosphorus from laying hens given another microbial phytase product at 250 FTU/kg diet was reported by Van der Klis et al. (1996; 1997). Similarly, Balander and Flegal (1996) reported decreased phosphorus excretion in market turkeys given Allzyme Phytase. These fecal phosphorus reductions from laying hens and market turkeys with Allzyme Phytase supplemented feeds were similar to the fecal phosphorus reductions found in pigs given another phytase feed supplement (Simons et al., 1990), layers (Simons and Versteegh, 1992; 1993), and broilers (Yi et al., 1996a,b).
PHYTASE INFLUENCE ON BROILER PERFORMANCE
Several researchers have demonstrated improved production performance of broiler chickens given feeds supplemented with a phytase product. Improved growth performance, assessed by increased body weight, feed intake and better feed efficiency, has been reported consistently in both chickens (Schoner et al., 1991; 1993; Broz et al., 1994; Sebastian et al., 1996a,b) and turkeys (Qian et al., 1996a,b). Qian et al. (1996b) further demonstrated improved performance of broilers given supplemental dietary phytase, which was correlated with improved bone growth and mineralization. This response was attributed to increased retention of certain minerals and nutrients in addition to phytase-liberated phytate-phosphorus, and this conclusion was supported by earlier research on phytase (Swick and Ivey, 1990; Simons et al., 1992; Hoppe and Schwarz, 1993).
The efficacy of supplemented microbial phytase is dependent upon dietary calcium (Ca) levels. Sebastian et al. (1996b) demonstrated that phytase-supplemented broiler diets with calcium concentrations greater than 1.25% caused less retention of phosphorus and nitrogen. Part of this problem is associated with the fact that calcium and magnesium (Mg) precipitate phytate as an insoluble Ca-phytate or Mg-phytate in the intestine inhibiting the release of phytate phosphorus by phytase (McQuaig et al., 1972; Scheideler and Sell, 1987; Sandberg et al., 1993). Qian et al. (1997) reported that phytase, vitamin D3 and calcium:total phosphorus ratios are important factors in degrading phytate phosphorus and improving dietary phosphorus and calcium utilization in broilers.
MANURE NUTRIENT MANAGEMENT IN CAGE-REARED VERSUS LITTER-REARED BROILER CHICKENS
Smith (1972) suggested that in the future broilers would be raised in cages in spite of the fact that at that time technology had not advanced to point where that practice was feasible. From the mid-1960s to the early-1980s many attempts were made to design cage facilities for the rearing of broiler chickens from hatch to market age. Unfortunately, none of these early cage systems allowed economical broiler production because of the overall poor performance (Lloyd et al., 1971; Lloyd and Chaloupka, 1972; Andrews and Goodwin, 1973; Andrews et al., 1975; Bayer et al., 1976). Recently, the Josef Kuhlman Company of Germany (Farmer Automatic of America) designed a cage rearing system (Broilermatic® System) that appears to have eliminated many of the early problems associated with cage rearing of broilers (Havenstein et al., 1998).
The advent of this new caging system holds promise for poultry producers in Europe and in other locations where land prices are so expensive as to preclude the establishment of conventional poultry rearing facilities, but production of chickens in cages poses a new problem in manure nutrient management. In a dynamic microenvironment established in poultry litter, the content of phosphorus can be approximately five-fold the concentration of phosphorus found in fresh raw manure (Zublena et al., 1993; Vest et al., 1994).
The litter serves as a source of nutrients that can be recycled by the chicken as it practices coprophagy. While little attention is given to this important source of nutrients for the chicken, when the bird is taken off litter and placed in cages where it does not have access to the nutrients eliminated in feces, one must be concerned about performance of the bird and weigh the potential negative effects of caging against the availability of nutrients in the litter. Additional studies, however, must be conducted to determine the minimum levels of nutrients required by broilers in a cage environment. The Broilermatic® System provided a unique opportunity to study methods of reducing manure disposal volume and nitrogen volatilization while at the same time providing much needed information about minimizing fecal phosphorus and nitrogen output.
MATERIALS AND METHODS
A 2 × 4 factorially arranged completely randomized experimental design was used to test the effects of phytase on broiler chickens reared in replicate pens in conventional litter-covered floor pens and replicate pens in cage rows in the cage-rearing facility (Broilermatic® System). There were 40 chickens in each of the 64 pens (e.g. 8 rows of 8 cages with 320 birds per cage row) in the Broilermatic® System cage-rearing facility and 40 chickens in each of the 32 pens in the conventional broiler house. Using this design, a total of 3,840 broiler chickens were used in the trial. Sexes were grown separately, thus providing a total of 1920 males and 1920 females in this experiment. A total of 1280 chickens (640 males and 640 females) were housed in the conventional house and 2560 (1280 males and 1280 females) were assigned to the Farmer Automatic Cage House.
Allzyme Phytase (Alltech, Inc.) is derived from an Aspergillus niger. Additional side activities of the Allzyme Phytase included cellulase, protease, xylanase and acid phosphatase. Allzyme Phytase was incorporated as a dry powder into mash feeds at 11.27 PTU/g or 2 lbs/ton. The broilers were given North Carolina Agricultural Research Service diets (Table 1) for ad libitum consumption on the following schedule: starter diet, day 0-18 (3177 kcal ME/kg, 22.5% crude protein), grower diet, 18 to 35 days of age (3168 kcal ME/kg, 19.5% crude protein), and finisher diet, 35 to 42 days of age (3160 kcal ME/kg, 17.4% crude protein). Conventional feeds (NRC requirements) normally contain 0.59, 0.58, or 0.41% total phosphorus with 0.79, 0.80, or 0.46% calcium in the starter and grower. However in these studies, the starter and grower test diets were formulated to contain 0.5%, 0.4% or 0.3% available phosphorus with 1.0%, 0.8% and 0.6% total calcium. This was accomplished via manipulation of the dicalcium phosphate (inorganic phosphorus) concentration in each diet.
Finisher diets were not supplemented with inorganic phosphorus (Table 1). Allzyme Phytase was blended dry into the 0.5, 0.4, and 0.3% diets at the level 11.27 PTU/g into all three diets. The influence of the Allzyme Phytase on serum concentrations of phosphorus and total calcium, weight gain, feed conversion, livability, and manure and litter phosphorus and nitrogen were determined. Manure phosphorus and nitrogen content were determined on the basis of pounds per ton of dry matter in the manure or litter and on the basis of g total phosphorus and nitrogen per kg of dry matter intake. Sampling for serum phosphorus and total calcium took place at 3 and 6 weeks of age. Fecal phosphorus and nitrogen content were determined weekly on fresh feces collected from the belts under the cages in the Farmer Automatic Cage house and in litter collected from ten random sites within each sampled pen in the conventional house. An AOAC-certified commercial laboratory (Woodson and Tenent, Goldston, NC) analyzed the litter and manure for phosphorus by a colorimetric procedure and nitrogen by Kjeldahl analysis.
Analyses of variance were conducted for each parameter; and the level of significance was set at P£ 0.05 or less depending upon the F values generated by the GLM procedure. When differences among treatments were found, the means were separated by least significant difference following the procedures of SAS (1994).
RESULTS AND DISCUSSION
PHYTASE AND PERFORMANCE
Allzyme Phytase supplementation to the diets of broiler chickens had minimal effects on body weights at six weeks of age of broilers grown in cages and in conventional housing (Table 2). Reducing the supplemental inorganic phosphorus and replacement with Allzyme Phytase did not significantly affect body weight of the cage-reared broilers. Feed conversion ratios (FCR) ranged between 1.73 (0.5% P) and 1.81 (0.5% P + Phytase), and likewise, supplementation of Allzyme Phytase for reduced available inorganic phosphorus did not significantly affect FCR. Mortality rates were not different among treatments and ranged between 2.81 % (0.5% P + Phytase) and 2.03 % (0.4% P and 0.3% P + Phytase).
In the conventional house, Allzyme Phytase added to diets with 0.5% and 0.4% available inorganic phosphorus resulted in slight but nonsignificant depressions in six-week body weights (Table 2), but broilers given 0.3% inorganic phosphorus plus Phytase had body weights slightly higher than those of broilers given the 0.5% available phosphorus diet without phytase. There was a clear trend in the conventional house toward improved FCR with addition of Allzyme Phytase in all treatment groups with the best FCR in groups with the lowest levels of available inorganic phosphorus. The FCR in the cage facility was improved overall by 28 points in comparison to the conventional house. Mortality rates in the conventional house ranged between 1.56 and 2.19% and did not differ among the treatments.
Mixed results have been observed with regard to body weight response of broilers fed phytase. In this study, a decrease was evident in caged broilers fed phytase-supplemented diets. In young broiler chickens, phytase is associated with improved digestibility of amino acids (McKnight, 1998). Kornegay (1996b) did not find improved body weight in broilers fed a finisher diet with phytase from three to seven weeks of age. However, McKnight (1996), summarizing the work of several scientific studies, reported that phytase reversed the negative impact of reducing dietary inorganic phosphorus content. Qian et al. (1997) reported that broilers fed very low levels of dietary phosphorus showed depressed weight gain, and even with 600 to 900 FTU phytase supplementation weight gain was not equivalent to controls given NRC-recommended phosphorus levels. Additionally, this reduced weight gain even with phytase was related to the ratio of dietary calcium to total available phosphorus in the diet. Calcium:total phosphorus ratios greater than 1.5: 1.0 were associated with reduced weight gain.
Nevertheless, in this experiment broilers in the conventional house demonstrated slight reductions in body weight when given the 0.5% and 0.4% phosphorus diets with phytase while broilers given the 0.3% phosphorus diet with phytase had six-week body weights slightly higher than controls (0.5% phosphorus diet without phytase). It is possible that broilers in the conventional house practiced coprophagy and were obtaining additional nutrients unavailable to the birds in the cage rearing facility. The improved FCR in broilers given the Allzyme Phytase was consistent with observations made when broilers were given other phytase products (Simons et al., 1992; McKnight, 1996).
Table 1. Composition of North Carolina Agricultural Research Service basal broiler diets.
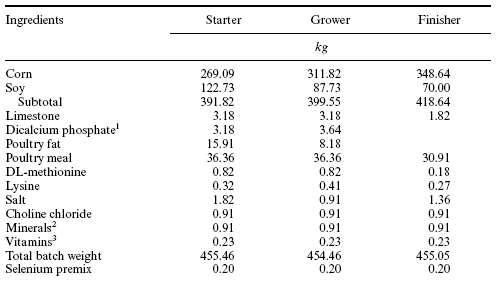
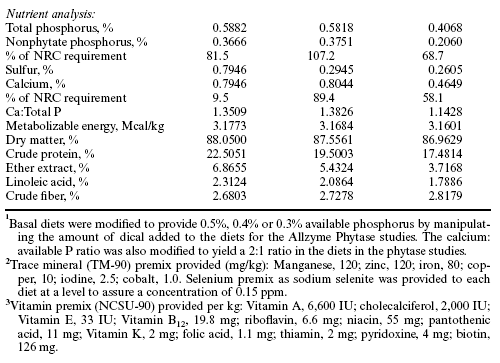
Table 2. Influence of Allzyme Phytase supplementation on production parameters for broiler chickens.
PHYTASE INFLUENCE ON SERUM CALCIUM AND INORGANIC PHOSPHORUS CONCENTRATIONS
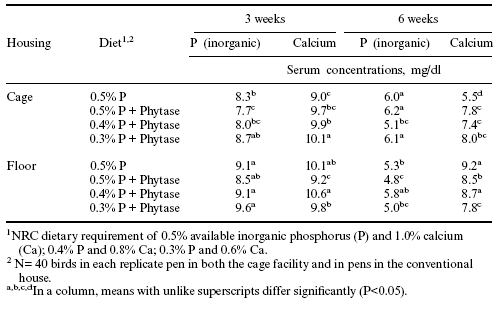
Allzyme Phytase and lower dietary levels of nonphytate phosphorus did not cause a decrease in serum phosphorus levels at either three or six weeks of age (Table 3). Serum total calcium concentrations were also unaffected in birds given diets where Allzyme Phytase was added to feed in which the calcium along with inorganic phosphorus levels were decreased.
Calcium:phosphorus (Ca:P) ratios were maintained at 2:1 for each of the experimental diets in this study. Serum Ca:P ratios ranged between 1.02 and 1.26 at three weeks of age. This was not a departure from ratios normally reported (Edens, 1976) even though values for both serum calcium and phosphorus were elevated at three weeks of age. At six weeks of age, the serum calcium and phosphorus concentrations were lower than at three weeks of age. However, nonphytate phosphorus and calcium in the finisher diets were 50% lower than in either the starter or grower diets, and as a result the serum levels of each were expected to be lower.
Nevertheless, serum Ca:P ratios at six weeks of age were higher than at three weeks and ranged between 0.92 and 1.80. At three weeks of age, serum Ca:P ratios were greater in the cage-reared broilers than in the floor-reared broilers, but at six weeks of age the cage-reared broilers had lower Ca:P ratios than the floor-reared broilers. The difference between the cage and floorreared birds at six weeks of age suggests the possibility of increased coprophagy in the floor-reared birds and a possible recycling of nutrients in the litter.
Table 3. Influence of Allzyme Phytase on serum inorganic phosphorus and total calcium concentrations in broilers.
PHYTASE INFLUENCE ON PHOSPHORUS CONTENT OF RAW MANURE AND LITTER
Allzyme Phytase supplementation of broiler diets significantly lowered phosphorus content (lbs/ton of manure dry matter) in raw manure from the birds in the cage facility (Table 4). This observation was most apparent in birds given the 0.3% available phosphorus diet compared to the 0.5% available phosphorus diet. Manure from birds given the 0.3% phosphorus diet contained from 6.6 to 32.2% less phosphorus during the feeding of the starter and grower diets. However, during the period when the finisher diet was given to the birds, there was no decrease in manure phosphorus in response to phytase supplements.
In the conventional house, birds given phytase without a concomitant reduction in dietary inorganic phosphorus showed increased rates of litter phosphorus accumulation (Table 4). Yet, with decreasing dietary inorganic phosphorus and supplemental phytase there was a clear decrease in litter phosphorus in the conventional house. In fact, at the end of the six week trial there was a 26.6% decrease in litter phosphorus accumulation in the conventional house in pens containing birds given the 0.3% phosphorus diet supplemented with the Allzyme Phytase.
Table 4. Influence of Allzyme Phytase supplementation on phosphorus content (lbs/ton dry matter) in raw manure from caged broilers and on accumulation of phosphorus in litter in the conventional house.
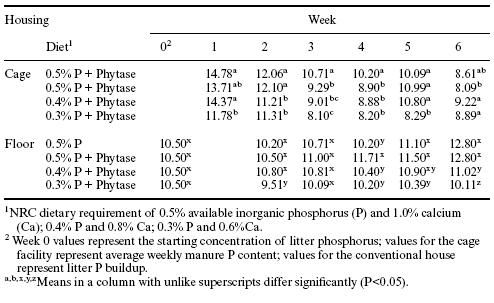
When manure phosphorus content was evaluated as a function of dry matter intake (Table 5) similar reductions were found when the birds were given the 0.3% and 0.4% available phosphorus starter and grower diets with Allzyme Phytase supplementation. Significant decreases in manure phosphorus ranged between 16.4 and 24% in the manure from birds given the 0.3% available phosphorus diets and housed in the cages.When given the 0.4% available phosphorus diets with phytase, manure phosphorus was reduced by 5.1 and 8.3% during the feeding of starter and grower, respectively.
During the final week of the study in the cage house, manure phosphorus increased in both the 0.3% and 0.4% available phosphorus groups and was no longer different from the control (0.5% available phosphorus). However, during the final week of the study in the conventional house there was a 26.6% reduction in litter phosphorus in pens where broilers were given the 0.3% available phosphorus diet with added phytase. A reason for the lack of a reduction in manure phosphorus in phytase-supplemented chickens in the cage facility at six weeks of age is not readily available.
However, Edwards (1993) and Van der Klis and Versteegh (1997a,b) have demonstrated that broiler chickens have the ability to degrade phytate phosphorus as they approach market age. The digestibility of phytate phosphorus can increase from 31% at 14 days of age to 38.2% at 25 days of age in broilers (Van der Klis and Versteegh, 1997a,b). At low levels of dietary inorganic phosphorus, phytate phosphorus becomes more available due to an adaptive increase in phytate digestibility (Moore and Veum, 1983). It has been noted that low levels of dietary phosphorus result in increased activity of intestinal phytase in chicks (Davies et al., 1970; McCuaig et al., 1972), explaining the observations made by Edwards (1993) and Van der Klis and Versteegh (1997a,b). Therefore, the increase in endogenous intestinal phytase in response to decreased dietary phosphorus with Allzyme Phytase activity added to the finisher diet, may have liberated more phytate phosphorus than the birds needed. As a result of increased phytase activity in the intestinal tract, excess inorganic and excess liberated phytate phosphorus were simply eliminated.
During the final week of this study no inorganic phosphorus was added to the experimental diets. The amounts of phosphorus eliminated were substantially lower in all treatments in the cage house and slightly higher in the litter in the conventional house (Tables 4 and 5). Therefore, on inspection of the data, it is clear that there were no treatment differences in phosphorus elimination in the cage house as one might expect given that there was no added inorganic phosphorus in the diet. However, in the conventional house, there were differences among dietary groups with less phosphorus elimination in the groups reared with lower inorganic phosphorus in their diets. This could indicate that there was recycling of nutrients in the floor-reared birds as they practiced coprophagy, or it may indicate that the birds on lower phosphorus intake were able to absorb more phosphorus from the intestinal tract. Answers to these problems are not apparent in the data from this study.
PHYTASE INFLUENCE ON NITROGEN CONTENT IN RAW MANURE AND LITTER
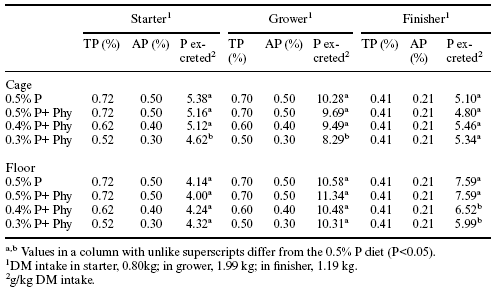
In both the cage facility and in the conventional house the addition of the Allzyme Phytase to normal and decreased phosphate diets was associated generally with lower output of manure nitrogen (lbs/ton dry matter in manure) when compared with the control (0.5% available phosphorus diet) (Table 6). This observation was apparent during the time when starter and grower diets were being fed to the broilers.Manure nitrogen content (lbs/ton dry matter in manure) was decreased from 4.8 to 24.6% in the cage facility and 8.0 to 21.5% in the conventional house. At six weeks, the litter nitrogen accumulation and raw manure nitrogen content was increased to the level of the control in birds given the phytase supplement (Table 6).
Table 5. Influence of Allzyme Phytase (Phy) supplementation in diets with varying concentrations of available phosphorus (AP%) on total phosphorus (TP%) excreted (g/kg dry matter intake (DMI) in raw manure from caged broilers and on accumulation of phosphorus in litter in the conventional house.
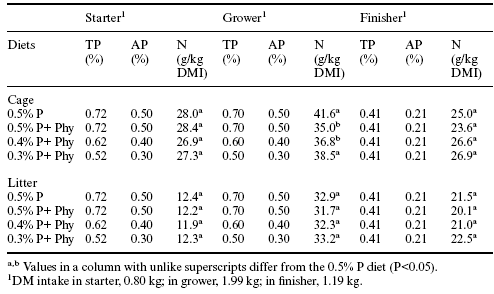
Litter nitrogen and raw manure nitrogen content were evaluated also on the basis of dry matter intake (Table 7). With this approach, no significant influence of phytase on litter nitrogen accumulation was observed. However, in the cage facility, there were significant reductions in manure nitrogen content in association with Allzyme Phytase supplementation in grower feeds (Table 7).
The changes in raw manure and litter nitrogen content associated with supplemental phytase are consistent with other observations. Caldwell (1992) demonstrated that phytate could inhibit proteolytic enzymes, and this inhibition of proteolysis could lead to decreased performance. Earlier reports had indicated that there was an improvement in protein utilization when dietary phytate was decreased and that dietary phytate was correlated positively with manure nitrogen output (Atwal et al., 1980; Knuckles et al., 1985). McKnight (1998) has shown that phytase can increase digestibility of amino acids leading to a reduction in manure nitrogen. In this case, where Allzyme Phytase was added to diets for broilers in cage or conventional housing there were significant transitory decreases in manure nitrogen (Tables 6 and 7).
However at six weeks of age there were nonsignificant increases in raw manure nitrogen or litter nitrogen from pens with broilers given the 0.3% phosphorus diet with Allzyme Phytase. This observation suggests that either other unaccounted influences may be interfering with protein hydrolysis in the intestinal tract or that the additional side activities of cellulase, protease, xylanase and acid phosphatase associated with Allzyme Phytase may have altered mucus secretion or even the microbial populations to an extent that additional nitrogen may have been eliminated in the feces of Allzyme Phytase-fed broilers. This hypothesis may be supported by the observation that there were slight depressions in body weight in all but one of the Allzyme Phytase treatments (Table 2). Additional studies are needed to understand the influence of phytate on protein digestibility in broilers.
Table 6. Influence of Allzyme Phytase on nitrogen content (lbs/ton of dry matter) in raw manure from caged broilers and on accumulation of nitrogen in litter in the conventional house.
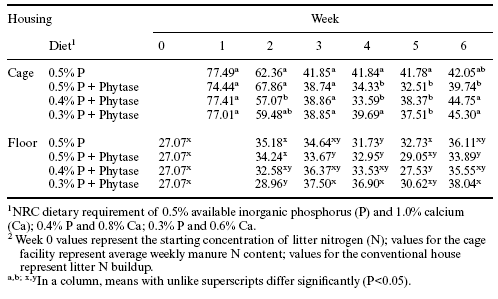
Table 7. Influence of Allzyme Phytase (Phy) supplementation in diets with varying concentrations of available phosphorus (AP%) on nitrogen (N) excreted in raw manure from caged broilers and on accumulation of phosphorus in litter in the conventional house.
SUMMARY
A worldwide concern about land application of poultry and livestock wastes is resulting in legislation in many countries that could result in national standards on pollution from phosphorus and nitrogen run-off into ground and surface waters. The use of microbial phytase in animal feeds has the potential to reduce significantly the concentration of phosphorus in manure by releasing the phytate phosphorus in the cell walls of feed grains thereby reducing the requirement for supplementation of inorganic phosphorus to animal diets. The phytate phosphorus meets the needs of the animal, and the resulting manure contains less inorganic phosphorus.
Using both a novel broiler cage rearing unit, the Farmer Automatic Broilermatic® System, and a conventional littercovered floor in a conventional broiler house, studies were conducted to ascertain production of phosphorus and nitrogen in manure from beneath broilers in cages and in built-up wood shavings litter. The use of Allzyme Phytase in the diet did not significantly affect performance even though with the lowest inorganic phosphorus diet body weights tended to be slightly lower than in controls with the NRC recommended dietary level of 0.5% available phosphorus.
In the conventional environment, FCR was improved between 2 and 5 points with phytase supplementation, but this was not evident in the cage house. The FCR in the cage house was improved by 28 points probably due primarily to the fact that the cage house was tunnel-ventilated using a Kool- Cell. There were no differences in mortality rates between the cage and conventional environments. However, there was a definitive trend toward decreased phosphorus in the wastes of broilers provided supplemental Allzyme Phytase with reductions in manure and litter phosphorus contents ranging to 32.2% in the raw manure from the cage house to 26.6% in litter in the conventional house. The nitrogen content of the manure was reduced in response to Allzyme Phytase suggesting that phytase supplementation can improve nitrogen utilization by the broiler chicken. These data suggest that the use of phytase in diets to replace inorganic phosphorus has a significant place in broiler management in both cage and conventional environments.
REFERENCES
Andrews, L.D. and T. L. Goodwin, 1973. Performance of broilers in cages. Poultry Sci. 52:723-728.
Andrews, L. D., G. S. Nelson, G. C. Harris, Jr. and T. L. Goodwin. 1975. Performance of five strains of broilers in a four tier cage system with plastic floors. Poultry Sci. 54:54-58.
Aoyagi, S. and D. Baker. 1995. Effect of microbial phytase and 1,25-dihydroxycholecalciferol on dietary copper utilization in chicks. Poultry Sci. 74:121-126.
Atwal, A. S., N. A. M. Eskin, B. E. McDonald and M. Vaisey-Genser. 1980. The effects of phytate on nitrogen utilization and zinc metabolism in young rats. Nutrition Reports Internat. 21:257-267.
Balander,R. J. and C. Flegal. 1996. The effect of using phosphatase enzyme on the performance of growing market turkeys and excreted phosphorus. Poultry Sci. 75(Suppl. 1):60 (Abstr).
Balander, R. J., and C Flegal. 1997. The effect of phytase on egg production and egg specific gravity in laying hens. Poultry Sci. 76(Suppl. 1):3 (Abstr).
Bayer, R. C., F. V. Muir, C. B. Chawan and A. T. Bryan. 1976. Infected feather follicles in cage reared broilers. Poultry Sci. 55:1194-1200.
Biehl, R. R., D. H. Baker and H. F. DeLuca. 1995. 1-a-hydroxylated cholecalciferol compounds act additively with microbial phytase to improve phosphorus, zinc, and manganese utilization in chicks fed soy-based diets. J. Nutr. 125:2407-2416.
Broz, J., P. Oldale, A. H. Perrin-Voltz,G. Rychen, J. Schulze and C. Simoes Nunes. 1994. Effect of supplemental phytase on performance and phosphorus utilization in broiler chickens fed low phosphorus diets without addition of inorganic phosphates. Br. Poult. Sci. 35: 273-280.
Caldwell, R. A. 1992. Effect of calcium and phytic acid on the activation of trypsinogen and the stability of trypsin. J. Agric. Food Chem. 40:43-46.
Cromwell, G. L. 1980. Biological availability of phosphorus in feedstuffs for swine. Feedstuffs 52(9):38-42.
Davies,M. I., G. M. Ritcey and I. Motzok. 1970. Intestinal phytase and alkaline phosphatase of chicks: Influence of dietary calcium, inorganic and phytate phosphorus and vitamin D3. Poultry Sci. 49:1280-1286.
Denbow, D. M., V. Ravindran, E. T. Kornegay, Z. Yi and R. M. Hulet. 1995. Improving phosphorus availability in soybean meal for broilers by supplemental phytase. Poultry Sci. 74:1831-1842.
Edens, F. W. 1976. Body temperature and blood chemistry responses in broiler cockerels given a single intravenous injection of Na+ or Ca++ before an acute heating episode. Poultry Sci. 55:2248-2255.
Edwards, H. M., Jr. 1993. Dietary 1, 25-dihydroxycholecalciferol supplementation increases natural phytate phosphorus utilization in chickens. J. Nutr. 123:567-577.
Erdman, Jr., J.W. 1979. Oil seed phytates: Nutritional implications. J. Am. Oil Chem. Soc. 56:736-741.
Havenstein,G. B., J. L. Grimes, P. R. Ferket, C. R. Parkhurst, F.W. Edens, J. Brake and J. H. van Middelkoop. 1998. Recent experiences with reduced or non-litter systems for growing broilers and turkeys. In: Proceedings: 1998 National Poultry Waste Management Symposium. Springdale, AR. October 19-21, 1998. Pages 225-240.
Heinzl,W. 1996. Technical specifications of Natuphos. In: BASF Technical Symposium: Phosphorus and Calcium Management in Layers. Atlanta, GA. January 23, 1996. Pages 21-36.
Hoppe, P. P. and G. Schwarz. 1993. Experimental approaches to establish the phosphorus equivalency of Aspergillus niger phytase in pigs. In: Proceedings of the 1st Symposium, Enzymes in Animal Nutrition. Kartause, Ittingen, Switzerland. Pages 187-192.
Knuckles, B. E., D. D. Kuzmicky and A. A. Betschart. 1985. The effect of phytate and partially hydrolyzed phytate on in vivo protein digestibility. J. Food Sci. 50: 1080-1082.
Kornegay, E. T. 1996a. Effect of phytase on the bioavailability of phosphorus, calcium, amino acids, and trace minerals in broilers and turkeys. In: BASF Technical Symposium: Phosphorus and Calcium Management in Layers. Atlanta, GA. January 23, 1996. Pages 39-68.
Kornegay, E. T. 1996b. Phytase supplementation of corn-soybean meal broiler diets. In: BASF Technical Symposium: Use of Natuphos Phytase in Poultry Nutrition andWaste Management. 1996 Carolina Swine Nutrition Conference, Raleigh, NC. November 12, 1996. Pages 63-72.
Lloyd,R.W.,G.W. Chaloupka andW. C.Kause. 1971. Incidence of feather follicle infection of broilers grown in cages. Poultry Sci. 50:1598.
Lloyd, R.W. and G.W. Chaloupka. 1972. The effect of plastic mat inserts in plastic coops on broiler performance. Poultry Sci. 51:1829-1830.
McKnight, W. F. 1996. Efficacy of microbial phytase in broiler grower diets. In: BASF Technical Symposium: Use of Natuphos Phytase in Poultry Nutrition andWaste Management. 1996 Carolina Swine Nutrition Conference, Raleigh, NC Pages 46-60.
McKnight,W. F. 1998. Nutritional alternatives to reduce nutrient loading enzymes. In: Proceedings: National Poultry Waste Management Symposium. Springdale, AR, October 19-20, 1998. Pages 160-168.
McQuaig, L. W., M. I. Davis and I. Motzok. 1972. Intestinal alkaline phosphatase and phytase in chicks: Effect of dietary magnesium, calcium, phosphorus, and thyroactive casein. Poultry Sci. 51:526-530.
Moore, R. J. and T. L. Veum. 1983. Adaptive increase in phytate digestibility by phosphorus-derived rates and the relationship of intestinal phytase and alkaline phosphatase to phytate utilization. Br. J. Nutri. 49:145-151.
Nelson, T. S., T. R. Shieh, R. J. Wodzinski and J. H. Ware. 1971. Effect of supplemental phytase on the utilization of phytate phosphorus by chicks. J. Nutr. 101:1289-1294.
Peeler, H. T. 1972. Biological availability of nutrients in feed. Availability of major mineral ions. J. Anim. Sci. 35:695-699.
Pointillart, A., C. Colin, C. Lacroix and J. Radisson. 1993. Reduction chez le pore en croissance de la supplementation en phosphorus mineral par l’utilization de cereales a activite elevee. Jour.Recher. Porcine, France, 25e: 233-238.
Qian, H., E. T. Kornegay and D. M. Denbow. 1996a. Phosphorus equivalence of microbial phytase in turkey diets as influenced by calcium to phosphorus ratios and phosphorus levels. Poultry Sci. 75:69-81.
Qian, H., H. P. Veit, E. T. Kornegay, V. Ravindran and D. M. Denbow. 1996b. Effect of supplemental phytase and phosphorus on histological and other tibial bone characteristics and performances of broilers fed semi-purified diets. Poultry Sci. 75:618-626.
Qian, H., E. T. Kornegay and D. M. Denbow. 1997. Utilization of phytate phosphorus and calcium as influenced by microbial phytase, cholecalciferol, and the calcium:total phosphorus ratio in broiler diets. Poultry Sci. 76:37-46.
Roberson, K. D. and H. M. Edwards. 1994. Effects of 1,25-dihydroxycholecalciferol and phytase on zinc utilization in broiler chicks. Poultry Sci. 73:1312-1326.
Sandberg, A. S., T. Larsen and B. Sandstrom. 1993. High dietary calcium levels decrease colonic phytase degradation in pigs. J. Nutr. 123:559-566.
SAS Institute. 1994. SAS® User’s Guide: Statistics. SAS Institute, Cary, NC, USA. Sauveur, B. 1993. Les phytases fongiques dans l’aliment des volailles. INRA Prod. Anim. 4:265-267.
Scheideler, S. E. and J. L. Sell. 1987. Utilization of phytate phosphorus in laying hens as influenced by dietary phosphorus and calcium levels. Nutr. Rep. Int. 35:1073-1081.
Schmitt, M. and G. Rehm. 1998. Fertilizing cropland with poultry manure. University of Minnesota Extension Service Bull. FO-5881-GO, Department of Soil, Water and Climate, Minneapolis-St. Paul, MN.
Schoner, B. R. J., P. P. Hoppe,G. Schwarz and H.Wiesche. 1991. Comparative effects of microbial phytase and inorganic phosphorus on performance and retention of phosphorus, calcium, and crude ash in broilers. J. Anim. Physiol. Anim. Nutr. 66:248-255.
Schoner, B. R. J., P. P. Hoppe, G. Schwarz and H.Wiesche. 1993. Effects of microbial phytase and inorganic phosphate in broiler chickens: performance and mineral retention at various calcium levels. J. Anim. Physiol. Anim. Nutr. 69:235-244.
Sebastian, S., S. P. Touchburn, E. R. Chavez and P. C. Lague. 1996a. The effects of supplemental microbial phytase on the performance and utilization of dietary calcium, phosphorus, copper, and zinc in broiler chickens fed corn-soybean diets. Poultry Sci. 75:729-736.
Sebastian, S., S. P. Touchburn, E. R. Chavez and P. C. Lague. 1996b. Efficacy of supplemental microbial phytase at different dietary calcium levels on growth and performance and mineral utilization of broiler chickens. Poultry Sci. 75:1516-1523.
Simons, P. C.M. and H. A. J. Versteegh. 1992. The effect of the addition of microbial phytase to layer feed on the technical results and skeleton and egg shell quality. Spelderholt Publication No. 568.
Simons, P. C.M. and H. A. J. Versteegh. 1993. The effect of the addition of low doses of microbial phytase to layer feed on the technical results and skeleton and egg shell quality. Spelderholt Publication No. 589.
Simons, P. C. M., H. A. J. Versteegh, A. W. Jongbloed, P. A. Keeme, P. Slump, K.D. Bos,M.G. E.Wolters, R. F. Beudeker andG. J. Verschoor. 1990. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br. J. Nutr. 64:525-540.
Simons, P. C. M., A. W. Jongbloed, H. A. J. Versteegh and P. A. Keeme. 1992. Improvement of phosphorus availability by microbial phytase in poultry and pigs. In: Proceeding Georgia Nutrition Conf. November 17-19, 1992, Atlanta, GA. Pages 100-107.
Smith,W. M, 1972.Whither cages in broilers? Poultry Digest. 31:76-77. Stillborn, H. 1998. Nutrition influences animal waste output. Feedstuffs, 70(18): 20:42-46.
Swick, R. A. and F. J. Ivey. 1990. Effect of dietary phytase addition on broiler performance in phosphorus deficient diets. Poultry Sci. 69 (Suppl. 1):133 (Abstr).
Van der Klis, J.D. and H. A. J. Versteegh. 1994. Effect of dietary measures to decrease phosphorus excretion by poultry. In: Nutrient Management Symposium: Seed and Feed FormulationResearch and Its Implications for Nutrient Management. Harrisburg, Pennsylvania. Pages 1-4.
Van der Klis, J. D. and H. A. J. Versteegh. 1997a. The degradation of inositol phosphates in broilers. 1. The effect of dietary calcium and absorbable phosphorus content. In: WPSA European Symposium on Poultry Nutrition. Faaborg, Denmark. August 24-28, 1997. Pages 465-467.
Van der Klis, J. D. and H. A. J. Versteegh. 1997b. The degradation of inositol phosphates in broilers. 2. The effect of age. In: WPSA European Symposium on Poultry Nutrition. Faaborg, Denmark. August 24-28, 1997. Pages 468-470.
Van der Klis, J.D., H. A. J. Versteegh and P. C. M. Simons. 1996. Natuphos in laying hen nutrition. In: BASF Technical Symposium. Phosphorus and Calcium Management in Layers. Carolina Poultry Nutrition Conference. Pages 71-83.
Van der Klis, J. D., H. A. J. Versteegh, P. C. M. Simons and A. K. Kies. 1997. The efficacy of phytase in corn-soybean meal-based diets for laying hens. Poultry Sci. 76:1535-1542.
Vest, L., B. Merka and W. I. Segars. 1994. Poultry Waste: Georgia’s 50 Million Dollar Forgotten Crop. Leaflet 206. Georgia Cooperative Extension Service, Athens, GA.
Williams, P. E. V. 1997. Poultry production and science: Future directions in nutrition. World’s Poultry Sci. J. 53:33-48.
Yi, Z., E. T. Kornegay and D. M. Denbow. 1996a. Improving phytate phosphorus availability in corn and soybean meal for broilers using microbial phytase and calculation of phosphorus equivalency values for phytase. Poultry Sci. 75:240-249.
Yi, Z., E. T.Kornegay andD. M. Denbow. 1996b. Supplemental microbial phytase improves zinc utilization in broilers. Poultry Sci. 75:540-546.
Zublena, J. P., J. C. Barker and T. A. Carter. 1993. Soil Facts: Poultry Manure as a Fertilizer Source. North Carolina Cooperative Extension Service, Raleigh, NC. 12
Authors: F. W. EDENS, C. R. PARKHURST and G. B. HAVENSTEIN
Department of Poultry Science, North Carolina State University, Raleigh, North Carolina, USA
Related topics
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post






.jpg&w=3840&q=75)