Performance of White Leghorn Chickens Breed Maintained at Haramaya University Poultry Farm and Implications for Sustainable Poultry Production
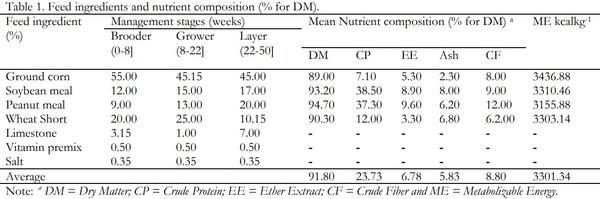
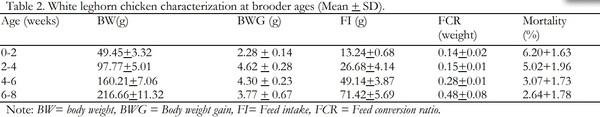
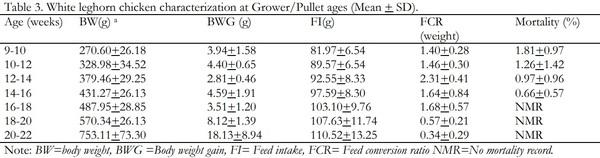
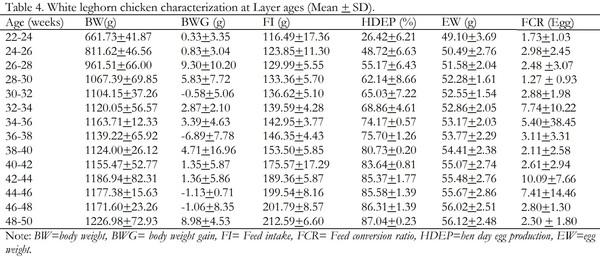
Indigenous chickens in Ethiopia are characterized by slow growth and egg production potential. As a result, poultry enterprises in the country entirely depend on exotic breeds, which are productive. The White Leghorn breed is the major one among the exotic breeds. Haramaya University Poultry farm is a source of the White Leghorn poultry breed in eastern Ethiopia. However, poor management and maintenance of the breed for too long without genetic improvement is a serious constraint to enhancing poultry production in the region. The objective of this study was, therefore, to evaluate the performance of White Leghorn breed maintained at Haramaya University poultry farm and establish their current reproductive and productive potential. The productive and reproductive performance of the breed was evaluated starting from hatching to 50 weeks of age. 576 eggs with an average size of 50.01 + 5.57g were randomly arranged into three replicates each consisting of 192 eggs. Then, a total of 363 hatched chicks were used and intensively raised on a deep litter system to evaluate body weight, feed intake, feed efficiency, body weight gain, mortality during brooder, grower and layer stages whereas egg weight and hen day egg production at layer ages were also determined. All data were analyzed using descriptive statistics. The results revealed that the mean hatchability, day old body weight, age at sexual maturity, weight at sexual maturity, hen-day egg production, and egg weight for the study breed were 70.32 + 4.08%, 33.48 + 0.84g, 154 days, 880.04 g, 70.35+3.22% and 53.47+2.39g, respectively. The average body weight and feed intake increased progressively during the brooder, grower and layer age. The highest weight gain was achieved during the grower age but the highest feed conversion ratio was observed during the layer stage. The mean mortality rates during the brooder and grower stages were 4.23+1.72 and 1.17+0.96, respectively. In conclusion, the White Leghorn breed at the university performed poorly with respect to most of the variables studied. Therefore, it is necessary to do more research to get insights into possible environmental and genetic factors that have contributed to the lower performance of the breed so as to address the constraints and enhance poultry production in the region.
Keywords: Brooder; Day-old chicks; Grower; Layer; Management stages; Performance; Reproductive; White Leghorn.

Abebe, H. 1992. Terminal report on the comparative evaluation of native chicken in the Hararge
Administrative Region and their crosses with the single comb White Leghorn. Mimeographed report, pp 22.
Abiola, S. S., Meshioye, O. O., Oyerinde, B. O. and
Bamgbose, M. A. 2008. Effect of Egg size on hatchability of broiler chiks. Archiva Zootechnica, 57:
83 -86.
Abraham, L. and Yayneshet, T. 2010. Performance of exotic and indigenous poultry breeds managed by smallholder farmers in northern Ethiopia. Livestock
Research for Rural Development, 22 (7).
Addis, G. and Malede, B. 2014. Effect of gene segregation on existing performance of chicken ecotypes in Ethiopia. Middle-East Journal of Scientific
Research, 21: 675-680.
Ahmad, F., Ahsan-ul-Haq, A.M., Hussain, J. and
Siddiqui, M.Z. 2010. Production performance of white leghorn hens under different lighting regimes.
Pakistan Veterinary Journal, 30: 21-24.
AOAC (Association of Official Analytical Chemist).
1990. Official Method of Analysis, 13th edition.
AOAC Arlington, Verginia, USA. pp. 12-98.
Barua, A., Devanath, S. C. and Hamid, M. A. 1992. A study on the performance of Rhode Island Red,
White Leghorn and their cross with Naked neck chicken. Asian-Australasian Journal of Animal Sciences,
5: 25-27.
Brannang, E. and Persson, S.1990. Ethiopian Animal
Husbandry. Uppsala, Sweden, pp127.
DeWitt, F. and Schwalbach, L.M.J. 2004. The effect of egg weight on the hatchability and growth performance of New Hampshire and Red Rhode
Island chicks. South African Journal of Animal Science,
34: 62-64.
Dominic, W., Rubin, C., Schutz, K., Kerje, S.,
Kindmark, A., Brandstrom, H., Andersson, L.,
Pizzari, T. and Per, J. 2012. Onset of Sexual
Maturity in Female Chickens is Genetically Linked to Loci Associated with Fecundity and a Sexual
Ornament. Reproduction in domestic animals, 47: 31-36.
Ewonetu, K. 2017. Growth Performance and Rearing
Costs of Fayoumi and White Leghorn Chicken
Breeds. East African Journal of Sciences, 11: 37-42.
Fahey, A. G., Marchant-Forde, R.M. and Cheng, H.W.
2007. Relationship between Body Weight and Beak
Characteristics in One-Day-Old White Leghorn
Chicks: Its Implications for Beak Trimming. Poultry
Science, 86:1312-1315.
Goto, T., Ishikawa, A., Onitsuka, S., Goto, N., Fujikawa,
Y., Umino, T., Nishibori, M. and Tsudzuki, M.
2011. Mapping quantitative trait loci for egg production traits in an F2 intercross of Oh-Shamo and White Leghorn chickens. Animal Genetics, 42:
634-641.
Haftu, K. 2016. A review of exotic chicken status, production performance and constraints in
Ethiopia. Asian Journal of Poultry Science, 10: 30-39.
Hossen, M.J. 2010. Effect of management intervention on the productivity and profitability of indigenous chickens under rural condition in Bangladesh.
Livestock research for rural development, 22(10).
Islam, M., Howlider, M.A.R., Kabir, F. and Alam, J.
2002. Comparative Assessment of Fertility and
Hatchability of Barred Plymouth Rock, White
Leghorn, Rhode Island Red and Rock hen.
International Journal of Poultry Science, 1: 85-90.
Itza-Ortiz, M.F., Peraza-Mercado, G., Castillo-Castillo,
Y., Rodriguez-Alarcon, C. A., Vital-Garcia, C. I.,
Jaramillo-Lopez, E. and Carrera-Chavez, J.M. 2016.
Productive Performance of White Leghorn Hens
Based on the Type of Housing during Rearing:
Floor versus Cage. Brazilian Journal of Poultry Science,
18: 543-548.
Jesus Eduardo, M.B., Mariano, J.G.A., Rosa, M.C.D.,
Omar, F.P.R., Jose, L.V., Xochitl, H.V., Guillermo,
T., Anita, M., Billy, M.H. and Silvia, C.D. 2013.
Effect of Time and Fatty Acid Composition in Eggs of White Leghorn Hens Supplemented with Tuna
Oil. Food and Nutrition Sciences, 4: 39-44.
Kebede, H., Urge, M. and Kebede, K. 2014. Effect of replacing maize with malted barley grain on fertility, hatchability, embryonic mortality and chick quality of white leghorn layers. Global Journal of Poultry
Farming and Vaccination, 2: 121-125.
Khalil, M. K., Al-Homidan, A. H. and Hermes, I. H.
2004. Crossbreeding components in age at first egg and egg production for crossing Saudi chickens with White Leghorn. Livestock Research for Rural
Development, 16 (1).
Cipav.org.co/Irrd/Irrd16/1/khal/161.htm.
Accessed on 16 November 2019.
Kutter, K. and Nitter, G. 1997. Effect of mating structure in purebred population on the estimation of cross breeding parameters. Journal of Animal
Breeding and Genetics, 114: 175-288.
Lukas, Z., Eva, T. and Ladislav, S. 2009. Effects of
Genotype, Age and Their Interaction on Egg
Quality in Brown-Egg Laying Hens. Acta Veterinaria
Brno, 78: 85-91.
Magomya, A.M, Kubmarawa, D., Ndahi, J.A, Yebpella,
G.G. 2014. Determination of Plant Proteins via the
Kjeldahl Method and Amino Acid Analysis: A
Comparative Study. International Journal of Scientific and Technology Research, 3:68-72.
Marangon, S. and Busani, L. 2006. The Use of
Vaccination in Poultry Production. International Office of Epizootics, 26: 265-274.
Malik, H.M., Haq, E.U. and Ahmad, F. 2008. Effect of
Age and Body Weight at Molting on the
Performance of Broiler Breeder Hens under
Environment Control House in Pakistan. Pakistan
Veterinary Journal, 28: 189-193.
Niknafs, S., Nejati-Javaremi, H., Mehrabani, Y. and
Fatemi, S.A. 2012. Estimation of Genetic
Parameters for Body Weight and Egg Production
Traits in Mazandaran Native Chicken. Tropical
Animal Health and Production, 44:1437-1443.
Niraj, K., Zinabu, N.B., Abebe, M.S. and Habtamu, T.
2014. Comparative Study of Performance of Rhode
Island Red and Bovans White under Intensive
Management in Mekelle, Ethiopia. International
Journal of Livestock Research, 4: 922-98.
Ochieng, J., Owuor, G., Bebe, B.O. and Ochieng, D.O.
2011. Effect of Management Interventions on
Productive Performance of Indigenous Chicken in
Western Kenya. Livestock research for rural development,
23(5).
Padhi, M.K., Chatterjee, R.N., Haunshi, S. and
Rajkumar, U. 2013. Effect of Age on Egg Quality in Chicken. Indian Journal of Poultry Science, 48: 122-
125.
Promila, N.K., Sajjan, S., Jyoti, S., Rakesh, V. and
Saurabh, B. 2017. Effect of Linseed Oil
Supplementation on Hen Day Egg Production,
Body Weight, Egg Shape Index, Economics and
Egg Quality in Layers. International Journal of Current
Microbiology and Applied Sciences, 6: 2005-2016.
Reddy, B.L.N., Panda, A.K., Reddy, M.R., Rao, S.V.R. and Praharaj, N.K. 2001. Studies on the Influence of Juvenile Growth Traits on Laying Performance in Egg Type Chickens. Indian Journal of Poultry
Science, 36: 290-293.
Rudra, P.G., Hasan, T., Rony, A. H., Adrian, G.,
Debnath, A., Islam, F. and Paul, P. 2018. Economic
Profitability of Broiler Farm Comparing the Two
Commercial Broiler Strain. Austin Journal of
Veterinary Science and Animal Husbandry, 5: 1045.
Sahin, E.H., Sengor, E., Cetingul, I.S. and Yardimci, M.
2009. Relationship between pre-incubation egg parameters from old breeder hens, egg hatchability and chick weight. Journal of Animal and Veterinary
Advances, 8: 115-119.
SAS (Statistical Analysis System). 2008. Statistical
Analysis User’s Guide: Statistics (2nd ed.). SAS institute, Inc.: North Carolina.
Scott, T.A. 2005. Variation in feed intake of broiler chickens. Recent Advances in Animal Nutrition in
Australia, 15: 237-244.
Shafik, A., El-Bayomi, K.H., Sosa, G.A.C. and Osman,
A. M. R. 2013. Effect of Crossing Fayoumi and
Rhode Island Red on Growth Performance, Egg and Reproductive Traits under Egyptian
Conditions. Benha Veterinary Medical Journal, 24: 11-
18.
Solomon, D. 2004. Egg production performance of local and White leghorn hens under intensive and rural household conditions in Ethiopia. Ethiopian Journal of science, 27:161-164.
Solomon, D. 2007. Comparative nutritive value of Atella and industrial brewers grains in chicken starter ration in Ethiopia. Livestock Research for Rural
Development, 19(1).
Sottnik, J. 2002. Climatical factors and their effect on production in animal housing. In: ASAE Annual
International Meeting/CIGR 15th World Congress;
Chicago, Illinois, USA: ASAE editors; 2002.
St-Pierre, N.R., Cobanov, B. and Schnitkey, G. X. 2003.
Economic Losses from Heat Stress by US
Livestock Industries. Journal of Dairy Science, 86: 52-
77.
Suarez, M. E., Wilson, H. R., Mather, F. B., Wilcox, C. J. and McPherson, B. N.1997. Effects of strain and age of the broiler breeder female on incubation time and chick weight. Poultry Science, 76: 10291036.
Talha, E. A., Yousuf, M. M., Ahmed M. E. and Hassabo,
A.A. 2011. Effect of fluctuating ambient temperature on the performance of laying hens in the closed poultry house. Research Opinions in Animal and Veterinary Sciences, 1: 254-257.
Tamasgen, N. 2015. The effect of feeding graded level of dried cafeteria food leftover on egg production and quality of white leghorn chickens. Journal of
Natural Sciences, Research, 5: 93-104.
Teketel, F. 1986. Studies on the meat production potential of some local strains of chicken in
Ethiopia. Ph.D. Thesis. Justus Liebig University
Giessen, pp. 210.
Thirunavukkarasu, P., Moorthy, M. and Viswanathan K.
2006. Body Weight and Egg Production
Performance of Induced Moulted White Leghorn
Layers. International Journal of Poultry Science, 5: 996-
1000.
Thirunavukkarasu, P., Moorthy, M. and Viswanathan, K.
2007. Body Weight Changes of Single Comb White
Leghorn Layers at Different Ages during Induced
Moult. International Journal of Poultry Science, 6: 858-
859.
Troianou, E., Huisman, J., Pemberton, J. M. and
Walling, C. A. 2018. Estimating selection on the act of inbreeding in a population with strong inbreeding depression. Journal of Evolutionary Biology,
31: 1815-1827.
Udeh, I. and Omeje, S.I. 2011. Growth and Short Term
Egg Production of Two Exotic (Layer Type) and the Local Chickens Compared with Their F1 Inbred
Progenies. International Journal of Poultry Science, 10:
221-224.
Ulmer-Franco, A.M., Fasenko,G.M., and O’Dea
Christopher, E.E. 2010. Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poultry Science, 89: 2735-2742.
Webster, A.B. and Czarick, M. 2000. Temperatures and performance in a tunnel-ventilated, high-rise layer house. Journal of Applied Poultry Research, 9: 118-129.
Wilson, H. R. 1991. Interrelationships of egg size, chick size, posthatching growth and hatchability. World
Poultry Science Journal, 47; 5-20.
Wiseman, J. 1987. Feeding of Non-Ruminant Livestock.
Butterworth and C Ltd, 370.
Yami, A. and Dessie, T. 1997. The Status of Poultry
Research and Development in Ethiopia, pp. 40-60.
In: Fifth National Conference of Ethiopian Society of Animal Production (ESAP), 15-17 May 1997,
Addis Ababa Ethiopia.
Yassin, H., Velthuis, A.G.J., Boerjan, M., Van Riel, J. and Huirne R.B.M. 2008. Field study on broiler eggs hatchability. Poultry Science, 87: 2408-2417.
Zewdu, W. and Berhan, T. 2013. The Effect of Feeding
Different Levels of Brewer’s Dried Grain Yeast
Mixture on the Performance of White Leghorn
Chicks. International Journal of Scientific and Research
Publications, 3: 1-5.








.jpg&w=3840&q=75)