Organic Chromium – an Exciting Beginning, a Promising Future
Published: January 2, 2008
By: MERLIN D. LINDEMANN (Courtesy of Alltech Inc.)
Published reports of research into the biochemical role of chromium (Cr) and its essentiality in the diet began to appear in the 1950s. Further pioneering work was reported in the 1960s with great attention paid to reports of insulin-related problems in patients receiving total parenteral nutrition and reversal of those problems with Cr inclusion in the administered solutions. Parenteral nutrition solutions traditionally used for the feeding of these patients were devoid of a supplemental Cr source. Several reports demonstrated that Cr supplementation either maintained proper glucose status or solved problems related to glucose tolerance in patients administered the parenteral solutions (Jeejeebhoy et al., 1977; Freund et al., 1979; Brown et al., 1986). These positive and dramatic results led to the unequivocal acceptance of Cr as a nutrient.
But, while Cr was accepted as a nutrient, the practical need for nutritional supplementation of many species for Cr were not accepted. Many of the various nutritional requirement species guidelines did not establish a requirement estimate for Cr. It was impossible to do so given the lack of research clearly demonstrating the situations which were Cr responsive and the levels of Cr needed to elicit those responses.
The excitement of recent research
There remains an inadequate amount of research to accurately define nutrient requirements for the various farm animal species. However, there have been many studies with a variety of farm animals that demonstrate amazing responses to the dietary addition of an organic form of Cr. Initial reports of positive effects were from the laboratory at Louisiana State University (Page et al., 1993) where researchers reported increased loin muscle area and decreased backfat of carcasses at market organic Cr was fed to growingfinishing pigs. In some of the studies the loin muscle area was increased more than 20% (7 cm2) and the tenth rib backfat decreased more than 25% (7 mm).
These positive results have been observed with Cr yeast also, but understanding of the factors controlling when a positive response is observed and the magnitude of response that might be expected is not yet complete. Consequently, the results are variable across research reports and intense research continues in this area.
Of even greater interest with pigs are the positive results that have been observed in reproduction. In the first reproductive study (Table 1), reported by Lindemann et al. (1995a), supplementation of 200 ppb Cr from Cr picolinate through two parities resulted in an increase of more than two pigs/litter born and weaned. The second reproductive study with Cr did not result in the same magnitude of response (Lindemann et al., 1995b) although the responses appeared to be developing and were understandable given the slightly different experimental procedure than the first study. Campbell (1998) has reported equally impressive responses to Cr supplementation, there being an increase in farrowing percentage from 79 to 92% with 200 ppb supplemental Cr in his first study. These reproductive results have also been observed with Cr yeast (Table 2).
Table 1. Effects of organic Cr on litter size in swine.
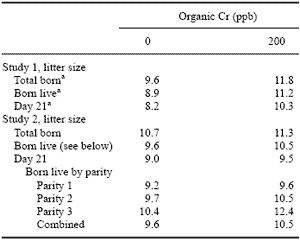
Adapted from Lindemann et al., 1995a,b. a Means differ (P < 0.05).
Table 2.Effects of Cr yeast (BioChrome) on sow performance.*

* A. Conolly, personal communication.
The three treatments were: no supplemental Cr, 200 ppb Cr from Cr yeast during lactation and the first 30 days of the next gestation, and 200 ppb Cr during lactation and all of gestation. The trial used only sows and was for one parity. Statistical evaluations were not performed on the results.
Exciting responses to organic Cr have also been observed in poultry. There have been positive effects noted in carcass composition and feed efficiency, as well as reductions in mortality in broiler studies. But even more interesting are the effects being observed in serum and egg yolk cholesterol in laying hens. Drs Lin and Lin (Table 3, 1997) have reported positive effects on serum and egg cholesterol and egg hatchability with supplementation of Cr yeast. Positive effects of a similar nature have also been observed in some studies with other forms of Cr.
Table 3. Effects of supplementation of Cr yeast (BioChrome) to layers.*
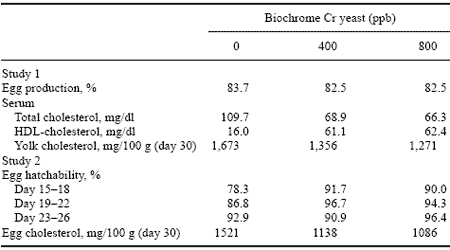
* Adapted from Lin and Lin, 1997.
HDL = high density lipoprotein.
Equally interesting results have been reported in exercising Thoroughbred horses supplemented with Cr yeast by Pagan et al. (1995). Benefits were observed in the metabolic response during periods of exercise on a high speed treadmill. During the exercise period, the supplementation of Cr resulted in observations of a reduction of plasma glucose (P < 0.05), a reduction of plasma lactate (P < 0.10), a reduction of plasma cortisol (P < 0.05, Figure 1), and an elevation of plasma triglycerides (P < 0.10). This type of response in a situation in which a type of stress exists is consistent with results in other species wherein the response to Cr is sometimes small or unobservable in a non-stress situation but is magnified under stress.
The effects of Cr on serum cortisol (as exhibited in the equine work) have also been observed in cattle during situations of stress (Chang and Mowat, 1992). This reduction in cortisol was associated with increased (P < 0.05) serum IgM and total immunoglobulins in calves fed certain diets. Another trial (Moonsie-Shageer and Mowat, 1993) with multiple levels of Cr (0, 200, 500, or 1,000 ppb) from high-Cr yeast demonstrated that the highest level of supplementation significantly increased growth rate by 29% and dry matter intake by 15% compared to unsupplemented control animals during the first 30 days in the feedlot. There also appeared to be a reduction in morbidity (sick calves that needed treatment) with Cr supplementation (43, 0, 24, and 24%, respectively); and serum cortisol levels at day 28 were decreased (69.4, 63.2, 58.6, and 47.3 nmol/l; P < 0.05) with the increasing levels of supplementation.
Additionally, dairy cows have demonstrated immune responses in late gestation and early lactation (Burton et al., 1993) to the supplementation of Cr from an amino acid chelate.
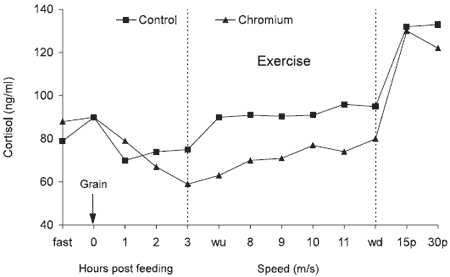
Figure 1.Effect of organic Cr on plasma cortisol values in Thoroughbred horses (Pagan et al., 1995). (wu = warm up; wd = warm down).
Cr nutrition has also been associated with adrenal compounds other than cortisol. Specifically there has been the observation of changes in the level of the adrenal steroid DHEA(dehydroepiandrosterone) in response to Cr supplementation.
DHEA, androstenedione, and DHEA-sulfate are adrenal precursor steroids for testosterone and estradiol. In women these adrenal precursors supply 50% of the androgenic hormone requirements. After menopause, they become the only source of this biological activity. Further, it has been postulated that hyperinsulinemia promotes macrovascular heart disease in part by reducing DHEA and DHEA-sulfate levels (Nestler et al., 1992).
These facts and postulates related to DHEA together led Evans et al. (1995) to examine the relationship between Cr, DHEAand calcium metabolism in postmenopausal women. In the study, pretrial DHEA and estrogen levels were comparable to other women aged 40 to 60 while urinary calcium was also elevated. After taking 400 μg of Cr as Cr picolinate for 60 days, plasma insulin decreased 37%, plasma glucose decreased 26%, and DHEAincreased 24% while calcium excretion decreased by 50%. The interrelationships among Cr, insulin, adrenal steroids, renal function, bone metabolism and, potentially, atherosclerosis are very exciting.
Mode(s) of action
The mode of action of Cr to elicit these responses is still a subject of debate and research. Some of the effects of Cr are well known while others are less clear. The initial observations of Cr effects were related to glucose metabolism in rats in which a factor in yeast was isolated that facilitated better glucose utilization. That factor was termed the glucose tolerance factor (GTF) and Cr was determined to be an integral part of it. Humans refractory to insulin administration subsequently responded to Cr supplementation (Jeejeebhoy et al., 1977; Freund et al., 1979; Brown et al., 1986), thus establishing clear relationships among glucose, insulin, and Cr that remain a subject of research to the present.
Clearly, the best known function of GTF is stimulation of the action of insulin in Cr-deficient tissue (Mertz, 1969). Insulin is a hormone that promotes anabolic and inhibits catabolic processes in muscle, liver, and adipose tissue. Effects on the efficiency of activity of endogenous insulin are a means whereby Cr is affecting some of the observed responses. The positive effects of Cr supplementation on insulin sensitivity of growing pigs has been demonstrated with Cr picolinate (Evock-Clover et al., 1993; Amoikon et al., 1995), Cr yeast (Guan et al., 1997), and Cr propionate (Matthews et al., 1997).
Evans and Bowman (1992, Table 4) have demonstrated an increase in amino acid and glucose uptake by rat skeletal muscle cells due to preculturing of the cells with Cr from Cr picolinate. This alteration of nutrient uptake was associated with alterations in insulin parameters and was Cr form-specific.
These observations may explain the effects on glucose tolerance as well as the improvement in muscling observed in some animal studies. Any potential improvement in amino acid uptake by muscle cells could be of benefit to total protein deposition. More recent work by Davis et al. (1996) suggested that the mechanism whereby Cr is involved with insulin is via the activation of a membrane phosphotyrosine phosphatase by a low molecular weight Cr-binding protein that is released concomitant with the insulin response to a meal.
Effects on insulin sensitivity have also been observed in mature pigs. In the first reproductive study involving Cr supplementation (Lindemann et al., 1995a), blood samples taken in mid-gestation demonstrated very clear differences in tissue responsiveness to insulin (Table 5) with those gilts receiving Cr having greater tissue sensitivity to insulin. Garcia et al. (1997; Table 6) recently confirmed these effects on tissue sensitivity to insulin in pregnant gilts and added the observation that oxytocin is also affected by Cr and, based just on numerical response, there could be reason to further evaluate effects of Cr supplementation on serum progesterone levels.
Alterations in insulin sensitivity may also partially explain the reproductive responses to Cr. Exogenous insulin has been demonstrated to increase the frequency of luteinizing hormone pulses in restricted-fed gilts and has been reported to increase the ovulation rate of gilts (Britt et al., 1988). Research at Mississippi State University has also demonstrated increases in ovulation rate in gilts in response to pre-estrus administration of insulin (Cox et al., 1987) and in litter size at second farrowing in response to insulin administration during the period from weaning of the first litter to re-mating (Ramirez et al., 1994). While these responses have not always been repeatable, they have been demonstrated, and provide the possibility that the effects of Cr on insulin function or on other hormones (such as oxytocin and progesterone) associated with reproduction are a mechanism whereby Cr elicits its effects.
Table 4. Effect of Cr source on rat skeletal muscle cells.*
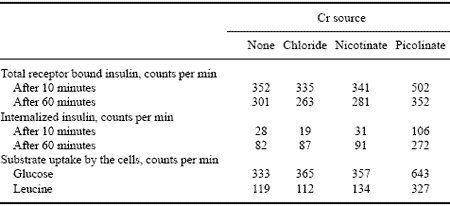
* Adapted from Evans and Bowman, 1992.
Table 5. Effect of supplemental Cr on serum glucose and insulin values in gestating gilts.*

* Adapted from Lindemann et al., 1995a.
‡ Glucose values are mg/100 ml and insulin values are pg/ml.
† Means differ (P < 0.05).
Table 6.Effects of Cr on glucose tolerance and ovarian and uterine function.*
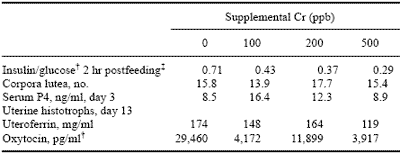
* Adapted from Garcia et al. (1997);
† Glucose values are mg/100 ml and insulin values are IU/ml; Cr form is picolinate.
‡ Means differ (P < 0.05).
Beyond the effects on insulin, Cr supplementation has clearly affected serum cortisol levels in multiple species. Whether this is a direct effect of Cr on the adrenal gland or an indirect effect via alterations of insulin is not determined.
However, the adverse effects of excess glucocorticoids such as cortisol are well known. The excesses inhibit fibroblasts, which can lead to a loss of collagen and connective tissue, which then can be manifest as easy bruising and poor wound healing. Negative effects can also be observed on bone formation and calcium absorption, as well as general growth and development in children. A host of other effects related to immunocompetence, renal function, cardiovascular function, and decreased libido are known. While these effects of gross glucocorticoid excess are known, whether the smaller moderations that result from Cr supplementation will have effects on long-term health and well-being are less certain.
The interrelationship of Cr with cholesterol should not be overlooked.
Demonstrations of Cr effect on cholesterol have been observed in poultry (as discussed earlier) as well as in humans. Cholesterol is a concern among the human population because of its relationship with, and implications in, cardiovascular disease. It does, however, play an important role as a structural membrane component and it is a precursor to the steroid hormones. The rate limiting enzyme in cholesterol synthesis is HMG-CoA (3-hydroxy-3- methylglutaryl coenzyme A) reductase. The mechanisms whereby Cr alters cholesterol levels and fractions is not totally understood. So, whether Cr elicits its effects via alterations in this enzyme level or activity or by another mechanism, the interrelationships of cholesterol to steroid hormones (of which progesterone may be altered with Cr supplementation) and to cardiovascular disease (which has been suggested to have the steroid DHEA as a ‘missing link’) are interesting.
A promising future
It should be noted that in many of the studies reported in which there has been a response to Cr supplementation, the subjects were in what could be considered stressful situations (i.e., heavy exercise, old age, having a preexisting health problem, pregnancy, crowding, etc.). Additionally, the fact that not all subjects in a given study were aided by Cr supplementation demonstrates that Cr is not a drug. A drug would have the general effect of altering some parameter in all subjects; Cr is a nutrient which, when supplemented, benefits humans or animals that are deficient while providing little or no benefit to those that are not deficient.
While this means that situations which the stockman considers ‘normal’ may not all respond to supplementation, it should still be noted that the research community does not totally understand what is indeed ‘normal good health or performance’ versus what is simply familiarity with performance or health in Cr-deficient subjects. The research does illustrate that a good portion of subjects suffering from some adverse health effects do so because of inadequate Cr nutrition. To the extent, then, that these situations result from the Cr status of the subject, they can be alleviated with proper Cr supplementation.
Anderson et al. (1993) demonstrated form-specific differences in tissue Cr content in rats supplemented with nine different forms of Cr. It is, therefore, necessary for proper experiments to be conducted to demonstrate the efficacy of the various forms of Cr. Additionally, the determination of an appropriate supplementation level for a mineral such as Cr is not an easy task. The NRC (1989) for humans states an assumption that the average ‘absorbability’ of Cr from normal dietary ingredients is 0.5%. If we assume the same for farm livestock, and if we assume that a normal diet might contain 1,500 μg of Cr per kg of diet, then the amount of Cr actually absorbed might be about 7.5μg/kg of diet.
Supplementing the diet with 750 μg of a hypothetical form of Cr that might be 2% available would provide 15 μg of Cr or double that provided by the normal diet. If the hypothetical form were 10% available it would provide 75 μg or 1,000% on an absorbed basis of that provided from the normal diet; if the form were 50% available it would provide 5,000% on an absorbed basis of that provided from the normal diet. The fact that the normal dietary constituents have such recognized low availability (0.5% in this example) results in an extremely large range of possible increase in availability (from 120 to 20,000% relatively) from supplemental sources. This precludes the establishment of a specific supplementation level for Cr in swine for all forms of Cr. The appropriate supplementation level will be formspecific.
The use of an inefficacious source of Cr would not be expected to cause problems because of the extremely low toxicity of trivalent Cr (lower than all other listed minerals such as selenium, copper, iodine, zinc, manganese AAFCO, 1995); but the use would represent added dietary expense without the expected economic return through improved health and performance.
Time/dose relationships relative to body mass may explain some of the inconsistencies in response to this point and may be fruitful areas of research.
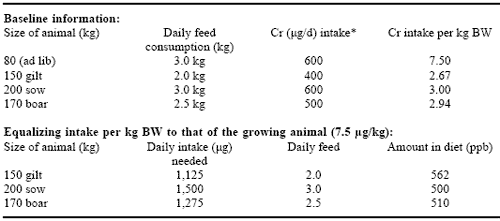
If time/dose relationships are indeed factors, then the level of supplemental Cr is an important consideration. Because muscle is a target organ for Cr, examination of Cr intake per unit of bodyweight may be an appropriate evaluation of supplementation adequacy. Muscle is the largest tissue mass in the body and, depending on the degree of depletion of Cr reserves in the body and the priority of different tissues for the Cr, it may take a period of time before all tissues receive an amount of Cr necessary to elicit a response. When feeding a nutrient at ppb levels, and when one of the target tissues is large, length of time of supplementation or dose level may be more critical than with some other nutrients with which the industry is more familiar. For example, because of limit feeding, reproducing swine are often fed a lower amount of vitamins and minerals per unit of bodyweight than finishing pigs and that is the case with Cr (Table 7).
Research currently being conducted will help to clarify some of the ambiguities that exist regarding Cr supplementation. It cannot be known what all of those revelations will be. However, in the development of our understanding to this point, certain patterns have emerged among a variety of interrelated physiological functions. Just as effects on glucose were soon linked to insulin and then aberrations in insulin function were found to respond to Cr supplementation, so it is probable that some other known problems in glucose and(or) insulin will be shown to respond to Cr supplementation.
Table 7. Effect of body size and physiological state on chromium intake.
* Daily intake (kg) × 200μCr/kg
Alterations in energy metabolism, lactate and cortisol levels suggest that endurance athletes would respond to Cr supplementation (e.g., sled dogs, race horses, jumpers in steeple chase, synchronized swimmers, and marathon runners).
The benefit would more likely occur in long-term performance versus short-term performance. Given that diabetics have vision problems and vascular problems, and given that a large number of adult-onset diabetics respond to Cr supplementation, there could be a reduction of vision problems, atherosclerotic events, and amputations in a segment of the human population.
Alterations in oxytocin could be related to birthing ease and the number of stillbirths resulting from birth trauma or anoxia in the process of birth.
Swine, humans, and dogs all are species that develop gestational diabetes and might respond to supplementation. Pregnancy toxemia in sheep, milk fever and fatty liver in cows are metabolic phenomena related to glucose and energy metabolism that may well respond to supplementation. Any metabolic abnormality that is related to insulin, glucose, cholesterol, and perhaps adrenal steroids may ultimately demonstrate a degree of responsiveness to Cr supplementation.
References
But, while Cr was accepted as a nutrient, the practical need for nutritional supplementation of many species for Cr were not accepted. Many of the various nutritional requirement species guidelines did not establish a requirement estimate for Cr. It was impossible to do so given the lack of research clearly demonstrating the situations which were Cr responsive and the levels of Cr needed to elicit those responses.
The excitement of recent research
There remains an inadequate amount of research to accurately define nutrient requirements for the various farm animal species. However, there have been many studies with a variety of farm animals that demonstrate amazing responses to the dietary addition of an organic form of Cr. Initial reports of positive effects were from the laboratory at Louisiana State University (Page et al., 1993) where researchers reported increased loin muscle area and decreased backfat of carcasses at market organic Cr was fed to growingfinishing pigs. In some of the studies the loin muscle area was increased more than 20% (7 cm2) and the tenth rib backfat decreased more than 25% (7 mm).
These positive results have been observed with Cr yeast also, but understanding of the factors controlling when a positive response is observed and the magnitude of response that might be expected is not yet complete. Consequently, the results are variable across research reports and intense research continues in this area.
Of even greater interest with pigs are the positive results that have been observed in reproduction. In the first reproductive study (Table 1), reported by Lindemann et al. (1995a), supplementation of 200 ppb Cr from Cr picolinate through two parities resulted in an increase of more than two pigs/litter born and weaned. The second reproductive study with Cr did not result in the same magnitude of response (Lindemann et al., 1995b) although the responses appeared to be developing and were understandable given the slightly different experimental procedure than the first study. Campbell (1998) has reported equally impressive responses to Cr supplementation, there being an increase in farrowing percentage from 79 to 92% with 200 ppb supplemental Cr in his first study. These reproductive results have also been observed with Cr yeast (Table 2).
Table 1. Effects of organic Cr on litter size in swine.

Adapted from Lindemann et al., 1995a,b. a Means differ (P < 0.05).
Table 2.Effects of Cr yeast (BioChrome) on sow performance.*

* A. Conolly, personal communication.
The three treatments were: no supplemental Cr, 200 ppb Cr from Cr yeast during lactation and the first 30 days of the next gestation, and 200 ppb Cr during lactation and all of gestation. The trial used only sows and was for one parity. Statistical evaluations were not performed on the results.
Exciting responses to organic Cr have also been observed in poultry. There have been positive effects noted in carcass composition and feed efficiency, as well as reductions in mortality in broiler studies. But even more interesting are the effects being observed in serum and egg yolk cholesterol in laying hens. Drs Lin and Lin (Table 3, 1997) have reported positive effects on serum and egg cholesterol and egg hatchability with supplementation of Cr yeast. Positive effects of a similar nature have also been observed in some studies with other forms of Cr.
Table 3. Effects of supplementation of Cr yeast (BioChrome) to layers.*

* Adapted from Lin and Lin, 1997.
HDL = high density lipoprotein.
Equally interesting results have been reported in exercising Thoroughbred horses supplemented with Cr yeast by Pagan et al. (1995). Benefits were observed in the metabolic response during periods of exercise on a high speed treadmill. During the exercise period, the supplementation of Cr resulted in observations of a reduction of plasma glucose (P < 0.05), a reduction of plasma lactate (P < 0.10), a reduction of plasma cortisol (P < 0.05, Figure 1), and an elevation of plasma triglycerides (P < 0.10). This type of response in a situation in which a type of stress exists is consistent with results in other species wherein the response to Cr is sometimes small or unobservable in a non-stress situation but is magnified under stress.
The effects of Cr on serum cortisol (as exhibited in the equine work) have also been observed in cattle during situations of stress (Chang and Mowat, 1992). This reduction in cortisol was associated with increased (P < 0.05) serum IgM and total immunoglobulins in calves fed certain diets. Another trial (Moonsie-Shageer and Mowat, 1993) with multiple levels of Cr (0, 200, 500, or 1,000 ppb) from high-Cr yeast demonstrated that the highest level of supplementation significantly increased growth rate by 29% and dry matter intake by 15% compared to unsupplemented control animals during the first 30 days in the feedlot. There also appeared to be a reduction in morbidity (sick calves that needed treatment) with Cr supplementation (43, 0, 24, and 24%, respectively); and serum cortisol levels at day 28 were decreased (69.4, 63.2, 58.6, and 47.3 nmol/l; P < 0.05) with the increasing levels of supplementation.
Additionally, dairy cows have demonstrated immune responses in late gestation and early lactation (Burton et al., 1993) to the supplementation of Cr from an amino acid chelate.

Figure 1.Effect of organic Cr on plasma cortisol values in Thoroughbred horses (Pagan et al., 1995). (wu = warm up; wd = warm down).
Cr nutrition has also been associated with adrenal compounds other than cortisol. Specifically there has been the observation of changes in the level of the adrenal steroid DHEA(dehydroepiandrosterone) in response to Cr supplementation.
DHEA, androstenedione, and DHEA-sulfate are adrenal precursor steroids for testosterone and estradiol. In women these adrenal precursors supply 50% of the androgenic hormone requirements. After menopause, they become the only source of this biological activity. Further, it has been postulated that hyperinsulinemia promotes macrovascular heart disease in part by reducing DHEA and DHEA-sulfate levels (Nestler et al., 1992).
These facts and postulates related to DHEA together led Evans et al. (1995) to examine the relationship between Cr, DHEAand calcium metabolism in postmenopausal women. In the study, pretrial DHEA and estrogen levels were comparable to other women aged 40 to 60 while urinary calcium was also elevated. After taking 400 μg of Cr as Cr picolinate for 60 days, plasma insulin decreased 37%, plasma glucose decreased 26%, and DHEAincreased 24% while calcium excretion decreased by 50%. The interrelationships among Cr, insulin, adrenal steroids, renal function, bone metabolism and, potentially, atherosclerosis are very exciting.
Mode(s) of action
The mode of action of Cr to elicit these responses is still a subject of debate and research. Some of the effects of Cr are well known while others are less clear. The initial observations of Cr effects were related to glucose metabolism in rats in which a factor in yeast was isolated that facilitated better glucose utilization. That factor was termed the glucose tolerance factor (GTF) and Cr was determined to be an integral part of it. Humans refractory to insulin administration subsequently responded to Cr supplementation (Jeejeebhoy et al., 1977; Freund et al., 1979; Brown et al., 1986), thus establishing clear relationships among glucose, insulin, and Cr that remain a subject of research to the present.
Clearly, the best known function of GTF is stimulation of the action of insulin in Cr-deficient tissue (Mertz, 1969). Insulin is a hormone that promotes anabolic and inhibits catabolic processes in muscle, liver, and adipose tissue. Effects on the efficiency of activity of endogenous insulin are a means whereby Cr is affecting some of the observed responses. The positive effects of Cr supplementation on insulin sensitivity of growing pigs has been demonstrated with Cr picolinate (Evock-Clover et al., 1993; Amoikon et al., 1995), Cr yeast (Guan et al., 1997), and Cr propionate (Matthews et al., 1997).
Evans and Bowman (1992, Table 4) have demonstrated an increase in amino acid and glucose uptake by rat skeletal muscle cells due to preculturing of the cells with Cr from Cr picolinate. This alteration of nutrient uptake was associated with alterations in insulin parameters and was Cr form-specific.
These observations may explain the effects on glucose tolerance as well as the improvement in muscling observed in some animal studies. Any potential improvement in amino acid uptake by muscle cells could be of benefit to total protein deposition. More recent work by Davis et al. (1996) suggested that the mechanism whereby Cr is involved with insulin is via the activation of a membrane phosphotyrosine phosphatase by a low molecular weight Cr-binding protein that is released concomitant with the insulin response to a meal.
Effects on insulin sensitivity have also been observed in mature pigs. In the first reproductive study involving Cr supplementation (Lindemann et al., 1995a), blood samples taken in mid-gestation demonstrated very clear differences in tissue responsiveness to insulin (Table 5) with those gilts receiving Cr having greater tissue sensitivity to insulin. Garcia et al. (1997; Table 6) recently confirmed these effects on tissue sensitivity to insulin in pregnant gilts and added the observation that oxytocin is also affected by Cr and, based just on numerical response, there could be reason to further evaluate effects of Cr supplementation on serum progesterone levels.
Alterations in insulin sensitivity may also partially explain the reproductive responses to Cr. Exogenous insulin has been demonstrated to increase the frequency of luteinizing hormone pulses in restricted-fed gilts and has been reported to increase the ovulation rate of gilts (Britt et al., 1988). Research at Mississippi State University has also demonstrated increases in ovulation rate in gilts in response to pre-estrus administration of insulin (Cox et al., 1987) and in litter size at second farrowing in response to insulin administration during the period from weaning of the first litter to re-mating (Ramirez et al., 1994). While these responses have not always been repeatable, they have been demonstrated, and provide the possibility that the effects of Cr on insulin function or on other hormones (such as oxytocin and progesterone) associated with reproduction are a mechanism whereby Cr elicits its effects.
Table 4. Effect of Cr source on rat skeletal muscle cells.*

* Adapted from Evans and Bowman, 1992.
Table 5. Effect of supplemental Cr on serum glucose and insulin values in gestating gilts.*

* Adapted from Lindemann et al., 1995a.
‡ Glucose values are mg/100 ml and insulin values are pg/ml.
† Means differ (P < 0.05).
Table 6.Effects of Cr on glucose tolerance and ovarian and uterine function.*

* Adapted from Garcia et al. (1997);
† Glucose values are mg/100 ml and insulin values are IU/ml; Cr form is picolinate.
‡ Means differ (P < 0.05).
Beyond the effects on insulin, Cr supplementation has clearly affected serum cortisol levels in multiple species. Whether this is a direct effect of Cr on the adrenal gland or an indirect effect via alterations of insulin is not determined.
However, the adverse effects of excess glucocorticoids such as cortisol are well known. The excesses inhibit fibroblasts, which can lead to a loss of collagen and connective tissue, which then can be manifest as easy bruising and poor wound healing. Negative effects can also be observed on bone formation and calcium absorption, as well as general growth and development in children. A host of other effects related to immunocompetence, renal function, cardiovascular function, and decreased libido are known. While these effects of gross glucocorticoid excess are known, whether the smaller moderations that result from Cr supplementation will have effects on long-term health and well-being are less certain.
The interrelationship of Cr with cholesterol should not be overlooked.
Demonstrations of Cr effect on cholesterol have been observed in poultry (as discussed earlier) as well as in humans. Cholesterol is a concern among the human population because of its relationship with, and implications in, cardiovascular disease. It does, however, play an important role as a structural membrane component and it is a precursor to the steroid hormones. The rate limiting enzyme in cholesterol synthesis is HMG-CoA (3-hydroxy-3- methylglutaryl coenzyme A) reductase. The mechanisms whereby Cr alters cholesterol levels and fractions is not totally understood. So, whether Cr elicits its effects via alterations in this enzyme level or activity or by another mechanism, the interrelationships of cholesterol to steroid hormones (of which progesterone may be altered with Cr supplementation) and to cardiovascular disease (which has been suggested to have the steroid DHEA as a ‘missing link’) are interesting.
A promising future
It should be noted that in many of the studies reported in which there has been a response to Cr supplementation, the subjects were in what could be considered stressful situations (i.e., heavy exercise, old age, having a preexisting health problem, pregnancy, crowding, etc.). Additionally, the fact that not all subjects in a given study were aided by Cr supplementation demonstrates that Cr is not a drug. A drug would have the general effect of altering some parameter in all subjects; Cr is a nutrient which, when supplemented, benefits humans or animals that are deficient while providing little or no benefit to those that are not deficient.
While this means that situations which the stockman considers ‘normal’ may not all respond to supplementation, it should still be noted that the research community does not totally understand what is indeed ‘normal good health or performance’ versus what is simply familiarity with performance or health in Cr-deficient subjects. The research does illustrate that a good portion of subjects suffering from some adverse health effects do so because of inadequate Cr nutrition. To the extent, then, that these situations result from the Cr status of the subject, they can be alleviated with proper Cr supplementation.
Anderson et al. (1993) demonstrated form-specific differences in tissue Cr content in rats supplemented with nine different forms of Cr. It is, therefore, necessary for proper experiments to be conducted to demonstrate the efficacy of the various forms of Cr. Additionally, the determination of an appropriate supplementation level for a mineral such as Cr is not an easy task. The NRC (1989) for humans states an assumption that the average ‘absorbability’ of Cr from normal dietary ingredients is 0.5%. If we assume the same for farm livestock, and if we assume that a normal diet might contain 1,500 μg of Cr per kg of diet, then the amount of Cr actually absorbed might be about 7.5μg/kg of diet.
Supplementing the diet with 750 μg of a hypothetical form of Cr that might be 2% available would provide 15 μg of Cr or double that provided by the normal diet. If the hypothetical form were 10% available it would provide 75 μg or 1,000% on an absorbed basis of that provided from the normal diet; if the form were 50% available it would provide 5,000% on an absorbed basis of that provided from the normal diet. The fact that the normal dietary constituents have such recognized low availability (0.5% in this example) results in an extremely large range of possible increase in availability (from 120 to 20,000% relatively) from supplemental sources. This precludes the establishment of a specific supplementation level for Cr in swine for all forms of Cr. The appropriate supplementation level will be formspecific.
The use of an inefficacious source of Cr would not be expected to cause problems because of the extremely low toxicity of trivalent Cr (lower than all other listed minerals such as selenium, copper, iodine, zinc, manganese AAFCO, 1995); but the use would represent added dietary expense without the expected economic return through improved health and performance.
Time/dose relationships relative to body mass may explain some of the inconsistencies in response to this point and may be fruitful areas of research.
If time/dose relationships are indeed factors, then the level of supplemental Cr is an important consideration. Because muscle is a target organ for Cr, examination of Cr intake per unit of bodyweight may be an appropriate evaluation of supplementation adequacy. Muscle is the largest tissue mass in the body and, depending on the degree of depletion of Cr reserves in the body and the priority of different tissues for the Cr, it may take a period of time before all tissues receive an amount of Cr necessary to elicit a response. When feeding a nutrient at ppb levels, and when one of the target tissues is large, length of time of supplementation or dose level may be more critical than with some other nutrients with which the industry is more familiar. For example, because of limit feeding, reproducing swine are often fed a lower amount of vitamins and minerals per unit of bodyweight than finishing pigs and that is the case with Cr (Table 7).
Research currently being conducted will help to clarify some of the ambiguities that exist regarding Cr supplementation. It cannot be known what all of those revelations will be. However, in the development of our understanding to this point, certain patterns have emerged among a variety of interrelated physiological functions. Just as effects on glucose were soon linked to insulin and then aberrations in insulin function were found to respond to Cr supplementation, so it is probable that some other known problems in glucose and(or) insulin will be shown to respond to Cr supplementation.
Table 7. Effect of body size and physiological state on chromium intake.

* Daily intake (kg) × 200μCr/kg
Alterations in energy metabolism, lactate and cortisol levels suggest that endurance athletes would respond to Cr supplementation (e.g., sled dogs, race horses, jumpers in steeple chase, synchronized swimmers, and marathon runners).
The benefit would more likely occur in long-term performance versus short-term performance. Given that diabetics have vision problems and vascular problems, and given that a large number of adult-onset diabetics respond to Cr supplementation, there could be a reduction of vision problems, atherosclerotic events, and amputations in a segment of the human population.
Alterations in oxytocin could be related to birthing ease and the number of stillbirths resulting from birth trauma or anoxia in the process of birth.
Swine, humans, and dogs all are species that develop gestational diabetes and might respond to supplementation. Pregnancy toxemia in sheep, milk fever and fatty liver in cows are metabolic phenomena related to glucose and energy metabolism that may well respond to supplementation. Any metabolic abnormality that is related to insulin, glucose, cholesterol, and perhaps adrenal steroids may ultimately demonstrate a degree of responsiveness to Cr supplementation.
References
AAFCO. 1995. Guidelines for contaminant levels permitted in mineral feed ingredients. In: Association of American Feed Control Officials Official Publication. p. 221.
Amoikon, E.K., J.M. Fernandez, L.L. Southern, D.L. Thompson, Jr., T.L.Ward and B.M. Olcott. 1995. Effect of chromium tripicolinate on growth, glucose tolerance, insulin sensitivity, plasma metabolites, and growth hormone in pigs. J. Anim. Sci. 73:1123.
Anderson, R.A., N.A. Bryden and M.M. Polansky. 1993. Form of chromium effects tissue chromium concentrations. FASEB J. 7:A204.
Britt, J.H., J.D. Armstrong and N.M. Cox. 1988. Metabolic interfaces between nutrition and reproduction. Proc. 11th Int. Congr. Anim. Reprod. Artificial Insemination, Dublin, Ireland. pp.117–125.
Brown, R.O., S. Forloines-Lynn, R.E. Cross andW.D. Heizer. 1986. Chromium deficiency after long-term total parenteral nutrition. Dig. Dis. and Sci. 31:661.
Burton, J.L., B.A. Mallard and D.N. Mowat. 1993. Effects of supplemental chromium on immune response of periparturient and early lactation dairy cows. J. Anim. Sci. 71:1532.
Campbell, R.G. 1998. Chromium and its role in pig meat production. In: Biotechnology in the Feed Industry. Proceedings of the 14th Annual Symposium. (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Loughborough, Leics, UK.
Chang, X. and D.N. Mowat. 1992. Supplemental chromium for stressed and growing feeder calves. J. Anim. Sci. 70:559.
Cox, N.M., M.J. Stuart, T.G. Althen, W.A. Bennett and H.W. Miller. 1987. Enhancement of ovulation rate in gilts by increasing dietary energy and administering insulin during follicular growth. J. Anim. Sci. 64:507.
Davis, C., K. Michele, H. Sumrall and J.B. Vincent. 1996.Abiologically active form of chromium may activate a membrane phosphotyrosine phosphatase (PTP). Biochem. 35:12963.
Evans, G.W. and T.D. Bowman. 1992. Chromium picolinate increases membrane fluidity and rate of insulin internalization. J. Inorg. Biochem. 46:243.
Evans, G.W., G. Swenson and K.Walters. 1995. Chromium picolinate decreases calcium excretion and increases dehydroepiandrosterone (DHEA) in post menopausal women. FASEB J. 9:525.
Evock-Clover, C.M., M.M. Polansky, R.A. Anderson and N.C. Steele. 1993. Dietary chromium supplementation with or without somatotropin treatment alters serum hormones and metabolites in growing pigs without affecting growth performance. J. Nutr. 123:1504.
Freund, H., S. Atamian and J.E. Fischer. 1979. Chromium deficiency during total parenteral nutrition. J. Am. Med. Assoc. 241:496.
Garcia, M.R., M.D. Newcomb and W.E. Trout. 1997. Effects of dietary chromium picolinate supplementation on glucose tolerance and ovarian and uterine function in gilts. J. Anim. Sci. 75:82 (Abstr.).
Guan, X.F., J.L. Snow, P. Ku, J. Burton and N.L. Trottier. 1997. Effect of dietary chromium supplementation on plasma glucose kinetics in barrows and gilts. J. Anim. Sci. 75 (Suppl. 1):189.
Jeejeebhoy, K.N., R.C. Chu, E.B. Marliss, G.R. Greenberg and A. Bruce- Robertson. 1977. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am. J. Clin. Nutr. 30:531.
Lin, X. and F. Lin. 1997. Effects of organic chromium on reduction of yolk cholesterol and alleviation of heat stress in laying hens. Fujian Agricultural University, PRC.
Lindemann, M.D., C.M.Wood, A.F. Harper, E.T. Kornegay and R.A. Anderson. 1995a. Dietary chromium picolinate additions improve gain/feed and carcass characteristics in growing/finishing pigs and increase litter size in reproducing sows. J. Anim. Sci. 73:457.
Lindemann, M.D., A.F. Harper and E.T. Kornegay. 1995b. Further assessments of the effects of supplementation of chromium from chromium picolinate on fecundity in swine. J. Anim. Sci. 73 (Suppl. 1):185.
Matthews, J.O., L.L. Southern, J.M. Fernandez, A.M. Chapa, L.R. Gentry and T.D. Bidner. 1997. Effects of dietary chromium tripicolinate or chromium propionate on growth, plasma metabolites, glucose tolerance, and insulin sensitivity in pigs. J. Anim. Sci. 75 (Suppl. 1):187.
Mertz, W. 1969. Chromium occurrence and function in biological systems. Physiol. Rev. 49:163.
Moonsie-Shageer, S. and D.N. Mowat. 1993. Levels of supplemental chromium on performance, serum constituents and immune status of stressed feeder calves. J. Anim. Sci. 71:232.
National Research Council (NRC). 1989. Recommended Dietary Allowances, 10th Ed. National Academy of Sciences. National Academy Press, Washington, DC.
Nestler, J.E., J.N. Clore andW.G. Blackard. 1992. Dehydroepiandrosterone: the ‘missing link’ between hyperinsulinemia and atherosclerosis? FASEB J. 6:3073.
Pagan, J.D., T. Rotmensen and S.G. Jackson. 1995. Effect of chromium supplementation on metabolic response to exercise in Thoroughbred horses. In: Proceedings of the 14th Equine Nutrition and Physiology Symposium. Ontario, Jan 19–21, 1995.
Page, T.G., L.L. Southern, T.L. Ward and D.L. Thompson, Jr. 1993. Effect of chromium picolinate on growth and serum and carcass traits of growingfinishing pigs. J. Anim. Sci. 71:656.
Ramirez, J.L., N.M. Cox and A.B. Moore. 1994. Enhancement of litter size in sows by insulin administration prior to breeding. J. Anim. Sci. 72 (Suppl. 1):79 (Abstr.).
Author: MERLIN D. LINDEMANN
Department of Animal Sciences, University of Kentucky, Lexington, Kentucky, USA
Related topics
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post




.jpg&w=3840&q=75)