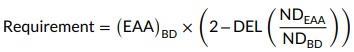1 INTRODUCTION
During the last decades, the productivity of laying hens has improved significantly, with higher number of eggs produced over the laying cycle and higher efficiency to utilize feed nutrients. This increase in productivity implies in a change in the requirements, and consequently, these hens become more demanding in nutrients, mainly amino acids (Elliot, 2008). Optimization of protein supply by understanding the amino acid needs of these hens requires a thorough understanding of the metabolic utilization of proteins and amino acids to provide a better feeding programme that suits better various environmental conditions and health status.
The efficiency of utilization of the dietary protein provided is dependent on the amount, composition and digestibility of its amino acids, which are required at specific levels by hens. According to the physiological needs, the absorbed amino acids (AA) should be available for protein synthesis of tissues, due to variation of individual tissue growth, age and performance; the ideal ratio required for maintenance and performance undergoes changes (Wecke & Liebert, 2013). In the case of hens, this becomes more important since these birds will prioritize egg production rather than the synthesis of other body protein compounds.
For the purpose of modelling the protein utilization in birds, some researchers have been using nonlinear models (Samadi & Liebert, 2007a). In these studies, the asymptote of the exponential equation is frequently associated with the maximum nitrogen retention (NRmaxT), while the intercept is associated with the nitrogen requirement for maintenance (NMR). As NRmaxT is determined by the asymptotic value of the exponential function, the concept of “theoretical” is assigned to this parameter, since this value is not observed in practical conditions of production (Samadi & Liebert, 2007a). The NMR is obtained by the value that intercepts the y-axis of an exponential function between nitrogen excreted and ingested when considering a nitrogen intake equal to zero. Once these two parameters have been estimated for a given genotype, it can be used in mathematical models to determine the efficiency of utilization and requirement of amino acids
The same researchers developed a way to determine the ideal amino acid ratios using the efficiency of utilization of the amino acids studied (Dorigam et al., 2017; Dorigam, Sakomura, Sünder, & Wecke, 2015; Pastor, Wecke, & Liebert, 2013; Wecke & Liebert, 2013). In addition, this procedure combines the concepts involved in the deletion method proposed by Wang and Fuller (1989) in which it is possible to determine the ideal ratio of amino acids through the changes in nitrogen retention with the individual amino acid deletions. Another approach can be applied from the deletion method to obtain the ideal amino acid ratio, which was proposed by Rollin, Mambrini, Abboudi, Larondelle, and Sadasivam (2003) based on an equation to determine the requirements of amino acids. Although it was suggested that the utilization of a limiting EAA is well described by a nonlinear model (Fatufe, Timmler, & Rodehutscord, 2004; Samadi & Liebert, 2008), the inflection point of a broken‐line model can predict minimal requirement values that are desirable for calculating EAA ratios (Baker, 2003).
Although the concept of ideal protein is widespread, some aspects still need to be improved. Updating the amino acid requirements for laying hens does not follow the same rate of development as those observed for broiler, and therefore, there is not so much information available to recommend or to conclude about the most appropriate ratio of amino acids for egg production in laying hens. Thus, the aim of this study was to determine the ideal amino acid ratios (IAARs) of essential amino acids in diets (Methionine + Cystine, Threonine, Tryptophan, Arginine, Valine, Isoleucine, Leucine, Phenylalanine + Tyrosine, Glycine + Serine and Histidine) for laying hens in peak of egg production (Hy‐line W36) making use of efficiency data calculated by the model parameters NMR and NRmaxT and the amino acid requirement proposed by Rollin et al (2003).
2 MATERIAL AND METHODS
This study was approved by the Ethics Committee on Animal Use of the Faculty of Agriculture and Veterinary Sciences, UNESP, Jaboticabal (no 9999/14).
Two nitrogen balance assays were conducted at the Laboratory of Poultry Science of the Faculty of Agriculture and Veterinary Sciences of Jaboticabal, São Paulo, Brazil. The assay 1 was performed to determine the nitrogen maintenance requirement (NMR) and the theoretical maximum nitrogen retention (NRmaxT), and the assay 2 was performed to determine the efficiency of amino acid utilization and the ideal amino acid ratio (IAAR) for Hy‐line W‐36 laying hens.
2.1 Assay 1: determination of the NMR and NRmaxT
2.1.1 Bird housing and experimental design
Fifty‐Six Hy‐line W36 laying hens were individually allotted in a completely randomized design with six treatments and eight replicates. The birds were housed in metabolic cages with wire floors, individual galvanized feeders and nipple drinkers installed in a facility with a negative-pressure ventilation system with controlled humidity and temperature. The management of the laying hens and the lighting programme followed the breeder’s guidelines (Hy-line W‐Management Guide2000).
2.1.2 Experimental diets
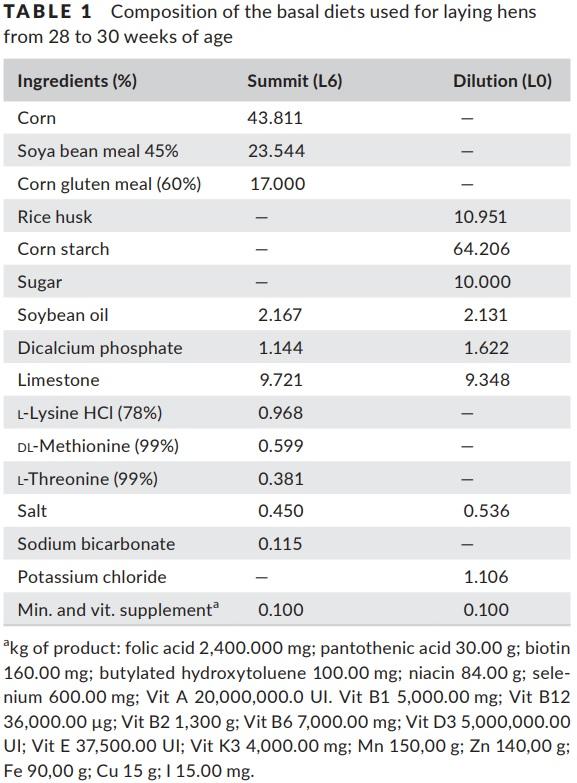
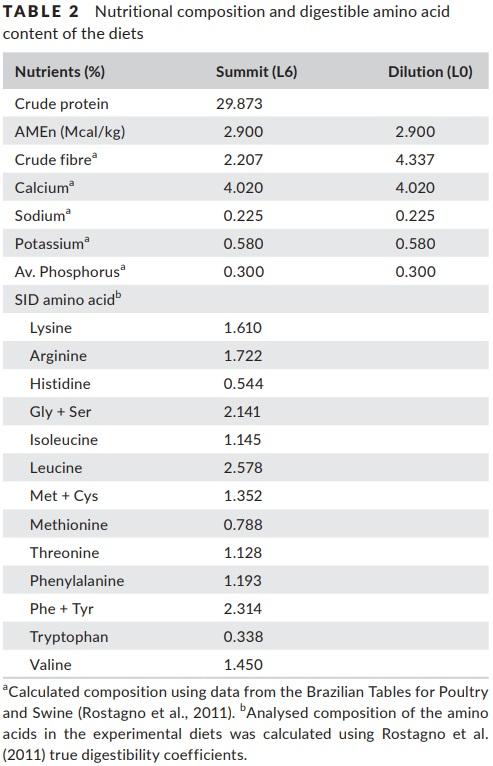
The treatments consisted of six graded levels of nitrogen in the diets (L1 = 8; L2 = 16; L3 = 24; L4 = 32; L5 = 40; and L6 = 48 g N/kg of feed), formulated using the dilution technique (Fisher & Morris, 1970). The dilution technique consisted of formulating two diets, one summit diet (L6) with high content of protein and another dilution diet (L0) free of protein and amino acids. The diet L6 was formulated using soyabean meal, corn gluten meal and industrial amino acids to meet the dietary concentration of 48 g N/ kg of feed. The L0 diet was formulated to meet the same nutritional level as the N6 diet, except for protein and amino acids. The composition of the diets is presented in Table 1. The experimental diets were obtained by diluting the L6 diet with the L0 diet, in the adequate proportion to obtain the intermediary levels of nitrogen while keeping the same essential amino acid ratios between the diets. The proportions of the high protein diet (L6) to protein‐free diet used in the preparation of the experimental diets were 15:85 for L1, 32:68 for L2, 49:51 for L3, 66:34 for L4, 83:17 for L5 and 100:0 for L6. The nutritional composition of the diets is presented in Table 2.
2.2 Assay 2: determination of the efficiency of amino acid utilization and ideal amino acid ratio
2.2.1 Bird housing and experimental design
Ninety‐Six Hy‐line W‐36 laying hens were individually allotted in a completely randomized design with 12 treatments and eight replicates. The hens were housed in same conditions described for assay 1.
2.2.2 Experimental diets
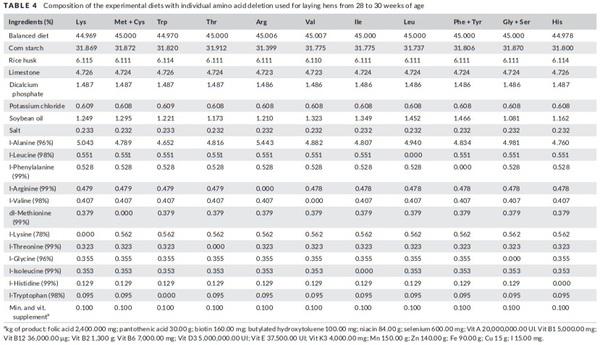
The treatments consisted of a balanced diet (BD) and eleven different diets deficient in one of the amino acid test (lysine, methionine + cystine, threonine, valine, isoleucine, arginine, tryptophan, leucine, histidine, glycine + serine and phenylalanine + tyrosine).
The BD was formulated to properly meet the nutritional requirements, and the ideal amino acid ratio (IAAR) provided by the Brazilian Tables for Poultry and Swine (Rostagno et al., 2011) for laying hens. The content of nitrogen and amino acids in the BD was supplied by corn, soyabean meal, corn gluten meal and a mixture of industrial amino acids (Table 3).
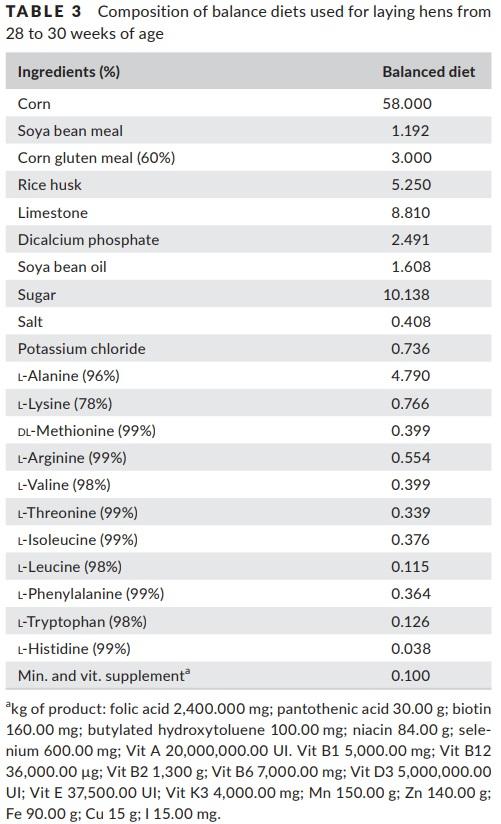
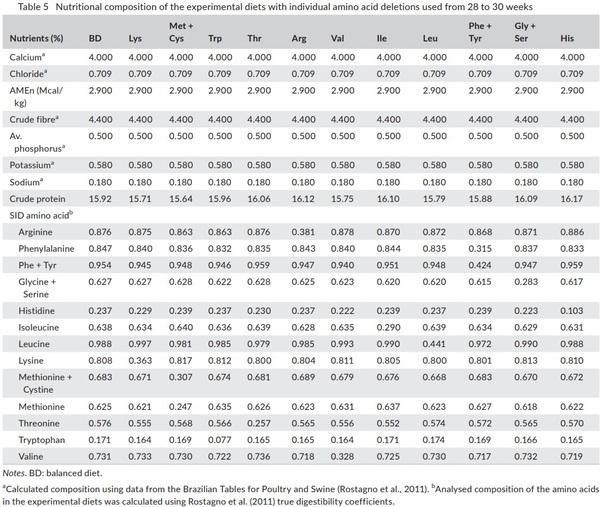
In order to formulate the deficient diets, the BD was diluted with cornstarch to meet 45% of amino acid requirement. The diets were supplemented with vitamin, mineral, fibre, energy and industrial amino acids to achieve the same levels of the BD, except for the amino acid to be evaluated in the treatment, which was deficient in 55% (Table 4). In the growth phases, the diets were formulated to meet 70% of amino acid requirements, while in the egg production to meet 45%, this difference is since laying hens are birds that are not in developing and have a great body fat reserve, these factors influence in the metabolism of the birds and a greater deficiency of amino acids is required to express response in a short period. The nutritional composition of the diets is presented in Table 5.
2.3 Experimental procedures
The selection and distribution of the laying hens in each experimental unit were performed according to body weight and egg production, based on recommendations of Sakomura and Rostagno (2016). In order to control the differences in the egg production and to confirm that all hens were laying eggs regularly, it was performed an individual control of the egg production during 14 days before starting the trial.
Each experimental period was divided into an adaptation period (5 days) and a period with total excreta and egg collection (10 days) to avoid that the birds were submitted to deficient diets for an extended period. The hens were weighed at the start and at the end of the experiment to measure body weight. The leftovers were weighed to measure daily feed intake. The excreta were collected, stored in plastic pots and weighed at the end of collection period to measure total excreta production. The egg production and egg weight were measured daily to calculate egg mass production. The egg mass was calculated by multiplying the egg weight by the egg production and then multiplied by the nitrogen content in the egg to calculate the amount of nitrogen deposited in egg mass (NEM). The NEM value is an important part of total N retention that should be considered in further estimation of the genetic potential as well as the utilization efficiency of dietary protein and amino acids for commercial laying hens (Dorigam et al., 2016, 2017).
2.4 Chemical analyses
The excreta and egg samples collected from each experimental unit were homogenized; an aliquot was removed and weighed in small plastic pots to freeze at −20ºC. Later, the samples were freeze-dried during a period of 72 hr at −80ºC under 800 mbar of pressure (Edwards® 501 Modulyo freeze dryer, West Sussex, UK). After the lyophilization, the dried samples were weighed to quantify the dry matter content. Subsequently, the dried samples were milled using a micro mill (IKA® A11 Basic Analytical Mill, Staufen, Germany). The nitrogen content of the diets, excreta and eggs was analysed in a nitrogen distiller (Kjeltec™ 8400 Foss; Foss, Hillerod, Denmark) using the Kjeldahl method (AOAC, 2005: method 2001.11). The total amino acid contents of the ingredients and the experimental diets were analysed by Evonik Industries using near-infrared spectroscopy (NIRs) and high-performance liquid chromatography (HPLC) respectively.
2.5 Statistical analysis
2.5.1 Assay 1: Determination of the NMR and NRmaxT
The nitrogen balance data were submitted to ANOVA using the general linear model (GLM) and then adjusted to an exponential model using PROC NLIN procedure in SAS (Statistic Analysis System, 9.1). The regression analyses between nitrogen intake (NI, mg/BWkg 0.67/ day) and nitrogen excretion (NEX, mg/BWkg 0.67/day) were performed to fit the exponential function and to determine the nitrogen maintenance requirement (NMR):
where NMR is the nitrogen maintenance requirement (mg/BWkg 0.67/ day), b is the slope of the exponential curve, and e is the base number of the natural logarithm (ln). The NMR was estimated by considering the intercept of the curve on the y-axis (NEX) when NI = 0.
The nitrogen deposition (ND, mg/BWkg 0.67/day) was determined by the difference between NI and the NEX. The nitrogen retention (NR, mg/BWkg 0.67/day) represents the total nitrogen utilization by the hen, and it was obtained by the sum of ND, NEM and NMR (Sünder, Wecke, & Liebert, 2010).
According to (Samadi & Liebert, 2007b, 2007a), the regression analyses between NI and NR were used to adjust the exponential model:
where NRmaxT is the maximum theoretical nitrogen retention (mg/ BWkg 0.67/day). The NRmaxT was estimated by a statistical procedure following several iterations by the Levenberg-Marquardt algorithm until the sum of the squares of the residual was minimized. This is an important parameter within the modelling procedure to derive AA requirements for defined graded levels to make use of the theoretical maximum within the scope of practical data.
2.5.2 Assay 2: determination of the efficiency of amino acid utilization and ideal amino acid ratio
The nitrogen balance data were analysed by a one-way ANOVA using a GLM procedure in SAS (Statistical Analysis System, version 9.1). Significant differences were tested using Dunnett’s test, and the pvalues < 0.05 were deemed statistically significant. The ideal amino acid ratio was determined from the limiting diets where the test amino acid was reduced in 55%; the protein quality (b) in each treatment was obtained by the equation of Samadi and Liebert (2008):
where NR is the nitrogen retention (mg/BWkg 0.67/day), NI is nitrogen intake (mg/BWkg 0.67/day), and NRmaxT is the maximum theoretical nitrogen retention (mg/BWkg 0.67/day) determined in assay 1.
The dietary protein quality (b) is linearly dependent on the concentration of the limiting amino acid in the feed protein (c). The calculation of c was made by the equation:
where LAAI is the amino acid test intake and NI is the nitrogen intake. It is possible to compare the model parameters bc−1 of an individual AA directly. The ratio between lysine efficiency (reference) and the efficiency of the limiting amino acid under study is utilized to derive IAAR, according to the calculation (Dorigam, 2016; Dorigam et al., 2016; Wecke & Liebert, 2013):
2.6 Determination of the amino acid requirement and ideal amino acid ratio
The amino acid requirement (g/kg DM) was determined using the following equation proposed by Rollin et al. (2003):
where (EAA)BD is the concentration of the considered EAA in the BD (g/kg DM), DEL is the deletion rate calculated by dividing the EAA concentration in the deficient diet by the EAA concentration in BD, NDEAA is the N deposition (mg N/BWkg 0.67/day) corresponding to the EAA diet, and NDBD is the N deposition observed on the BD (mg N/BWkg 0.67/day). This method is based on the assumption that N retention is a linear function of dietary essential amino acid content when a particular amino acid is limiting. An optimal balance between the EAA was derived by dividing the estimated requirement for each EAA by the estimated requirements for lysine (base lysine = 100).
3 RESULTS
3.1 Assay 1: Determination of the NMR and NRmaxT
The results obtained from the N balance trials with Hy‐line W‐36 laying hens from 28 to 30 weeks are presented in Table 6.
Increasing levels of protein in experimental diets did not influence BW, FI and b‐value (p > 0.05); however, as the dietary protein diets become higher, there was increase in NI, NEX, NEM and ND in evaluated period. It was observed a marked increase from L1 to L5 for NEM and from L1 to L4 for ND, after these levels a decrease in values could be observed.
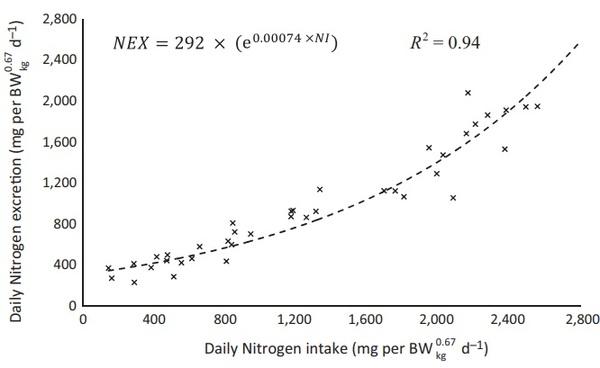
After fitting the exponential function between nitrogen intake and nitrogen excretion during a gradual increase in the protein supplied in peak of egg production, it was estimated the NMR of 292 mg/BWkg 0.67 from 28 to 30 weeks, as shown in Figure 1.
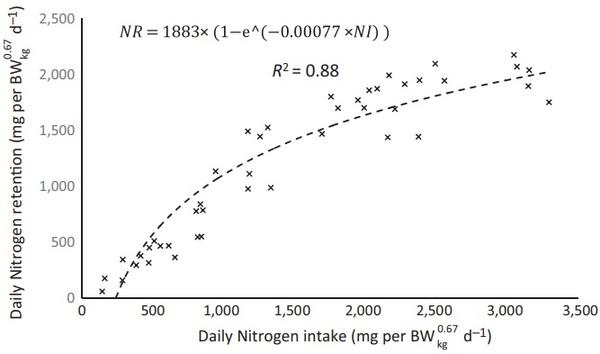
The maximum theoretical nitrogen retention was estimated in 1,883 mg/BWkg 0.67 for the period evaluated after the fitting of the exponential function between nitrogen intake and nitrogen retention, shown in Figure 2.
As demonstrated in the Figures 1 and 2, the values to NMR of 292 mg/BWkg 0.67, NRmaxT of 1,883 mg/BWkg 0.67 and maximum theoretical nitrogen deposition (NDmaxT) of 1,591 mg/BWkg 0.67 for Hyline W‐36 laying hens from 28 to 30 weeks were estimated.
3.2 Assay 2: determination of the efficiency of amino acid utilization, amino acid requirements and ideal amino acid ratio
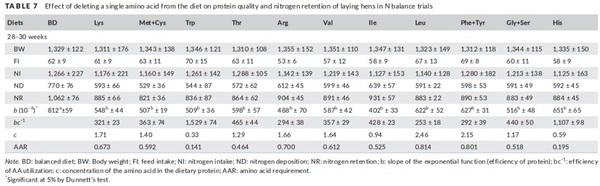
Summarized results of the N balance experiments are displayed in Table 7. The amino acid deficient diets had lower nitrogen balance data compared to BD. The deletion of essential amino acids reduced nitrogen retention and protein quality in different proportions depending on the deleted amino acid, however did not influenced in the BW, FI and NI.
As expected, deficient diets have lower protein quality, characterized by the decrease in the value of parameter b in relation to BD (p < 0.05), thus confirming the limiting position of the amino acids under study. The leucine, phenylalanine +tyrosine and histidine-deficient diet presented higher b-value compared to the other limiting diets, but still lower than the b-value of BD. The deletion of arginine and isoleucine significantly reduced the quality of dietary protein in relation to the other limiting diets evaluated.
When evaluating the efficiency of amino acid utilization, there is a gradual increase in bc−1 values from one period to the next. From the utilization efficiencies and amino acid requirements presented in Table 7 were determine the ideal ratios of the essential amino acid for Hy‐line W‐36 laying hens from 28 to 30 weeks by Goettingen and Louvain approaches presented in Table 8.
4 DISCUSSION
The aim of this study was to estimate the genetic potential for daily N retention (NRmaxT) and nitrogen maintenance requirement (NMR) of laying hens in peak of egg production (Hy‐line W‐36), to estimate the efficiency of utilization and to determine the ideal ratio of amino acids based on the combination of the deletion method and the Goettingen and Louvain approaches.
During the experimental periods (Assay 1 and 2), it was observed that the hens presented lower feed intake in relation to indicated by the Hy‐line W36 guideline, probably, due to the severity in the protein and amino acids reduction. This is a common characteristic of this type of study due to the formulation technique used to formulate experimental diets (Bendezu et al., 2015).
The levels of N used in this study provided responses for NI, NEX and NR that allowed estimation of the coefficients of Goettingen approach. The model parameter b as a measure of dietary protein quality is independent of the level of protein intake (Pastor et al., 2013; Wecke & Liebert, 2009). Thus, when the dietary protein quality remains unchanged, as observed in this study (Table 6), the individual N balance data can be utilized to estimate the threshold value of NRmaxT (Liebert, 2015).
The value of 292 mg/BWkg 0.67 for NMR represents the amount N that the birds should minimally consume to compensate the inevitable metabolic losses that independent of the intake (Samadi & Liebert, 2006a). The maximum theoretical nitrogen retention was estimated in 1,883 mg/BWkg 0.67, which is considered to be “theoretical” because the value estimated is not attainable in practical condition, even if the hens are bred under perfect conditions. However, this is an important parameter used for the modelling procedure of the amino acid requirements (Samadi & Liebert, 2006a, 2006b). Studies with the Goettingen approach for laying hens are few (Sünder et al., 2010), but there are already studies with broiler breeder hens (Dorigam et al., 2017), pullets (Bonato et al., 2015; Silva et al., 2013) and broilers in growth phase (Samadi & Liebert, 2008; Pastor et al., 2013; Santos et al., 2014; Daulat, Wecke, Sharifi, & Liebert, 2015, Wecke, Pastor, & Liebert, 2016).
Dorigam et al. (2017) working with broiler breeder hens of the Ross 308AP® genotype in production determined average NMR of 255 mg/BWkg 0.67/day and NRmaxT of 1,597 mg/BWkg 0.67/day; these values are, on average, 14% lower than those found in the study which can be attributed to differences in genotype, age, period of production and mainly by the physiological differences of the birds. The broiler breeders differ from commercial laying hens in terms of body growth, voracity, lipid reserves and, above all, the egg-laying frequency. This cycle normally completes with the laying of nine eggs (Nonis & Gous, 2013); on the other hand, the broiler breeders do not complete this cycle and present irregular laying, consequently a longer pause period and shorter cycles.
Samadi and Liebert (2006a) determined NMR of 252 mg/ BWkg 0.67/day for Cobb 500® broilers in the period from 10 to 85 days.
FIGURE 1 Estimation of the daily nitrogen requirement for maintenance (NMR) by fitting the exponential function between nitrogen intake (NI) and nitrogen excretion (NEX) for Hy‐line W‐36 laying hens. Observed (×) and predicted (̵ ̵ ̵) values from 28 to 30 weeks
FIGURE 2 Estimation of the maximum theoretical nitrogen retention (NRmaxT) for Hy‐line W‐36 laying hens based on the exponential fitting between nitrogen intake (NI) and nitrogen retention (NR). Observed (×) and ppredicted (̵ ̵ ̵) values from 28 to 30 weeks
Silva et al. (2013) and Bonato et al. (2015) found average NMR of 284 mg/BWkg 0.67/day and 307 mg/BWkg 0.67/day for Dekalb White® pullets in growth phase respectively. Daulat et al. (2015) determined average NMR of 310 mg/BWkg 0.67/day in a trial with naked neck broilers in growth phase, and these values are similar to found in this study. However, Wecke et al. (2016), Pastor et al. (2013) and Samadi and Liebert (2008) determined average NMR of 221 mg/BWkg 0.67 / day, 164 mg/BWkg 0.67/day and 240 mg/BWkg 0.67/day for Ross 308 ® broilers in the growth and finisher phases respectively. This variation between the values found can be justified by the use of different ingredients as protein sources.
The values determined to NRmaxT are difficult to compare with the data of other experiments due to variations in strains, age, feed intake and dietary characteristics. However, several studies with animals in growth (Daulat, et al., 2015; Dorigam, et al., 2014; Pastor, et al., 2013; Samadi & Liebert, 2008; Santos, et al., 2014; Wecke et al., 2016) presented similar characteristics where a decrease of threshold values with increasing age was observed, indicating that animals at maturity tend to have lower values and that the genetic potential is independent of the diet (Samadi & Liebert, 2008).
The estimation of NRmaxT and NMR is required for basic applications of the exponential model, as well as to define the genotype in terms of theoretical potential for N deposition, evaluate the value of the dietary protein quality based on the observed efficiency of the limiting amino acid and to determine of amino acid ideal ratio from the efficiency of the dietary amino acid (Liebert, 2017).
The ideal amino acid profile is based on the concept that while amino acid requirements change due to genetic or environmental factors, the relationships between them are slightly affected. Thus, once the amino acid ideal profile is determined, it is sufficient to determine the requirement of a single amino acid and the others can be calculated (Baker, 2003; Dari et al., 2005). The requirements determined by NRC (1994), Leeson and Summers (2005) and Rostagno et al. (2005, 2011, 2017, 2000) were estimated from a compilation of tests conducted with different diets, periods of production of the birds, strains and environmental conditions and then determined the amino acid ideal profile. Similarly, Coon and Zhang (1999) conducted five experiments to determine the amino acid requirements of laying hens and the ideal profile recommended by them is based on the average requirements determined in the five assays.
Bregendahl, Roberts, Kerr, and Hoehler (2008) followed the recommendations of Baker (2003) that, to ensure an accurate measure of the amino acid ideal profile, it is necessary that the experimental conditions be the same for all amino acid requirement assays. The same authors performed seven experiments, simultaneously, to determine the ideal ratio of methionine, methionine + cystine, threonine, tryptophan, valine, isoleucine and arginine. As in this study, a single basal diet was used; however, the synthetic amino acids were added to ensure that the amino acid tested was the first limiting. The Goettingen approach allows to model the amino acid requirement considering the sex, age and genotype (Samadi & Liebert, 2006b, 2007b, 2007a; Wecke & Liebert, 2009), so an adequate IAAR can be established from the corrected amino acid requirement. It is worth highlighting that the “requirement” determined by the Louvain approach is not used to make recommendations for nutritional levels in the diet, but rather to calculate of the ideal amino acid ratio.
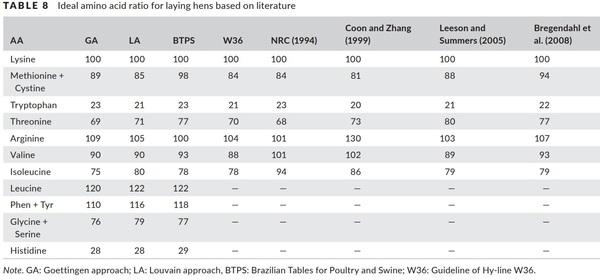
The ideal ratio determined by NRC (1994), Coon and Zhang (1999), Leeson and Summers (2005), Bregendahl et al. (2008), Guideline of Hy‐line W‐36 (2000) and Rostagno et al. (2017) and the obtained from the Goettingen and Louvain approaches are presented in Table 8.
The ideal ratio for Met+Cys:Lys presented larger divergence and the recommendation obtained by Goettingen and Louvain approaches were the same as that determined by Leeson and Summers (2005) and similar recommendations of the Guideline for Hy-line W‐36 (2000), NRC (1994) and Coon and Zhang (1999). The ideal rations determined by Bregendahl et al. (2008) and Rostagno et al. (2017) are much higher than the other findings in the literature.
The results for Tryptophan:Lys and Arginine:Lys are similar to literature, except the value found by Coon and Zhang (1999) for Arginine:Lys that was on average 25% higher than the others recommendations. The ideal ratio by the Goettingen and Louvain approaches for branched-chain amino acids, valine and isoleucine, was similar to the other studies. Highlights the recommendations of the NRC (1994) and Coon and Zhang (1999) that are on average 15% higher. The natural and periodic event of loss and replacement of feathers have different patterns and occur in response to a series of hormonal changes due to the photoperiod, which may justify the differences between the recommendations in the literature. The feathers are keratinized structures, rich in cystine, arginine and branched-chain amino acids, whose main function is to cover the body of the birds by protecting them and aiding in body thermoregulation (Leeson & Summers, 2005). According to Stilborn, Moran, Gous, and Harrison (1997), there is a change in the content of arginine, isoleucine, leucine and cystine throughout the life of the bird.
The Thr:Lys ratios found in the literature suffer small variations, except to the recommendations of the Guideline of Hy‐line W‐36 (2000) and NRC (1994), and the results obtained by Goettingen and Louvain approaches in this study that are lower compared to the recommendations of the other authors. Threonine is a hydroxyl amino acid that plays an important role with glycine and serine in the metabolism of porphyrin, acetyl CoA, pyruvate and uric acid. It is essential for the synthesis and catabolism of protein and is fundamental importance in maintaining intestinal health and integrity, and an important component in the development of feathers, participating in 4%–5% of its crude protein content (Kidd, 2000). It is found in concentrations close to 4.8% of egg protein, and its requirements depend on the age and crude protein level of the diet (Kidd & Fancher 2001).
The differences among the ideal amino acid ratio recommendations reflect the differences in how they were determined, and the requirements available in the literature were estimated from data compiled from a variety of experiments and therefore influenced by genetic, environmental, bird age, and different experimental conditions and basal diets.
In this study, NMR and NRmaxT values were determined, making possible to know the genetic potential or the physiological limit of the Hy‐line W36 strain, allowing to use strategies to optimize the performance of the birds. These values were used as parameters in nonlinear mathematical models to estimate the efficiency of utilization and the ideal ratio of the essential amino acids in a single assay based on the deletion method, providing more accurate values by minimizing the effects of the environment, diet and genotype.