Nutritional modulation of the antioxidant capacities in poultry: the case of vitamin E
Author details:
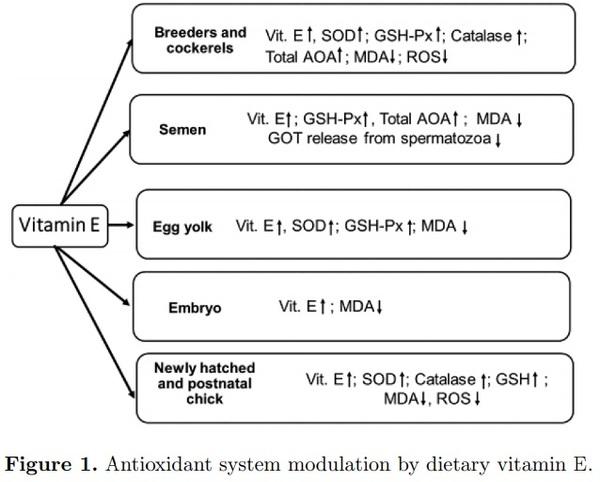
Commercial poultry production is associated with a range of stresses, including environmental, technological, nutritional, and internal/biological ones, responsible for decreased productive and reproductive performance of poultry. At the molecular level, most of them are associated with oxidative stress and damages to important biological molecules. Poultry feed contains a range of feed-derived and supplemented antioxidants and, among them, vitamin E is considered as the “headquarters” of the antioxidant defense network. It is well-established that dietary supplementation of selenium, vitamin E, and carotenoids can modulate antioxidant defenses in poultry. The aim of the present paper is to present evidence related to modulation of the antioxidant capacities in poultry by vitamin E. Using 3 model systems including poultry breeders/males, semen, and chicken embryo/postnatal chickens, the possibility of modulation of the antioxidant defense mechanisms has been clearly demonstrated. It was shown that increased vitamin E supplementation in the breeder’s or cockerel’s diet increased their resistance to various stresses, including high polyunsaturated fatty acids (PUFA), mycotoxin, or heat stress. Increased vitamin E supplementation of poultry males was shown to be associated with significant increases in α-tocopherol level in semen associated with an increased resistance to oxidative stress imposed by various external stressors. Similarly, increased vitamin E concentration in the egg yolk due to dietary supplementation was shown to be associated with increased α-tocopherol concentration in the tissues of the developing embryos and newly hatched chicks resulting in increased antioxidant defenses and decreased lipid peroxidation. Furthermore, increased vitamin E transfer from the feed to egg yolk and further to the developing embryo was shown to be associated with upregulation of antioxidant enzymes reflecting antioxidant system regulation and adaptation. The role of vitamin E in cell signaling and gene expression as well as in interaction with microbiota and maintaining gut health in poultry awaits further investigation.
Key words: nutritional modulation, antioxidant, vitamin E, oxidative stress, poultry.
Ara´ujo, I. C. S., M. B. Caf´e, R. A. Noleto, J. M. S. Martins, C. J.
Ulhoa, G. C. Guareshi, M. M. Reis, and N. S. M. Leandro. 2018.
Effect of vitamin E in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult. Sci. doi:10.3382/ps/pey439
Azzi, A. 2018. Many tocopherols, one vitamin E. Mol. Aspects Med.
61:92–103.
Barton, N. W. H, N. C. Fox, P. F. Surai, and B. K. Speake. 2002.
Vitamins E and A, carotenoids and fatty acids of the egg yolk of raptors. J. Raptor Res. 36:33–38.
Bilgili, S. F., J. A. Renden, and K. J. Sexton. 1985. Fluorometry of poultry semen: its application in the determination of viability, enzyme leakage, and fertility. Poult. Sci. 64:1227–1230.
Botsoglou, E., A. Govaris, D. Fletouris, and S. Iliadis. 2013. Olive leaves (Olea europea L.) and α-tocopheryl acetate as feed antioxidants for improving the oxidative stability of α-linolenic acidenriched eggs. J. Anim. Physiol. Anim. Nutr. 97:740–753.
Br´eque, C., P. Surai, and J. P. Brillard. 2003. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro.
Mol. Reprod. Dev. 66:314–323.
Brigelius-Floh´e, R. 2009. Vitamin E: the shrew waiting to be tamed.
Free Radic. Biol. Med. 46:543–554.
Cachia, O., J. E. Benna, E. Pedruzzi, B. Descomps, M. A. GougerotPocidalo, and C. L. Leger. 1998. α-Tocopherol inhibits the respiratory burst in human monocytes. Attenuation of p47phox membrane translocation and phosphorylation. J. Biol. Chem.
273:32801–32805.
Catignani, G. L., F. Chytil, and W. J. Darby. 1974. Vitamin E deficiency: immunochemical evidence for increased accumulation of liver xanthine oxidase. Proc. Natl. Acad. Sci. 71:1966–1968.
Cecil, H. C., and M. R. Bakst. 1993. In vitro lipid peroxidation of turkey spermatozoa. Poult. Sci. 72:1370–1378.
Cerolini, S., K. A. Kelso, R. C. Noble, B. K. Speake, F. Pizzi, and
L. G. Cavalchini. 1997. Relationship between spermatozoan lipid composition and fertility during aging of chickens. Biol. Reprod.
57:976–980.
Cerolini, S., P. F. Surai, B. K. Speake, and N. H. C. Sparks. 2005.
Dietary fish and evening primrose oil with vitamin E effects on semen variables in cockerels. Br. Poult. Sci. 46:214–222.
Cerolini, S., L. Zaniboni, A. Maldjian, and T. Gliozzi. 2006. Effect of docosahexaenoic acid and alpha-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology 66:877–886.
Cherian, G., F. W. Wolfe, and J. S. Sim. 1996. Dietary oils with added tocopherols: effects on egg or tissue tocopherols, fatty acids, and oxidative stability. Poult. Sci. 75:423–431.
Conrad, M., V. E. Kagan, H. Bayir, G. C. Pagnussat, B. Head, M.
G. Traber, and B. R. Stockwell. 2018. Regulation of lipid peroxidation and ferroptosis in diverse species. Gene. Dev. 32:602–619.
Danikowski, S., H. P. Sallmann, I. Halle, and G. Flachowsky. 2002.
Influence of high levels of vitamin E on semen parameters of cocks. J. Anim. Physiol. Anim. Nutr. 86:376–382.
Ebeid, T. A. 2012. Vitamin E and organic selenium enhances the antioxidative status and quality of chicken semen under high ambient temperature. Br. Poult. Sci. 53:708–714.
Eid, Y., T. Ebeid, and H. Younis. 2006. Vitamin E supplementation reduces dexamethasone-induced oxidative stress in chicken semen. Br. Poult. Sci. 47:350–356.
El-Hack, M. E. A., K. Mahrose, M. Arif, M. T. Chaudhry, I. M.
Saadeldin, M. Saeed, R. N. Soomro, I. H. Abbasi, and Z. U.
Rehman. 2017. Alleviating the environmental heat burden on laying hens by feeding on diets enriched with certain antioxidants (vitamin E and selenium) individually or combined. Environ. Sci.
Pollut. Res. 24:10708–10717.
Ewen, J. G., R. Thorogood, F. Karadas, A. C. Pappas, and P. F.
Surai. 2006. Influences of carotenoid supplementation on the integrated antioxidant system of a free living endangered passerine, the hihi (Notiomystis cincta). Comp. Biochem. Physiol. A Mol.
Integr. Physiol. 143:149–154.
Fernandes, J. I. M., H. L. F. Bordignon, K. Prokoski, R. C. Kosmann,
E. Vanroo, and A. E. Murakami. 2018. Supplementation of broiler breeders with fat sources and vitamin E: carry over effect on performance, carcass yield, and meat quality offspring. Arq. Bras.
Med. Vet. Zootec. 70:983–992.
Ga´al, T., M. M´ezes, R. C. Noble, J. Dixon, and B. K. Speake. 1995.
Development of antioxidant capacity in tissues of the chick embryo. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 112:711–
716.
Galli, F., A. Azzi, M. Birringer, J. M. Cook-Mills, M. Eggersdorfer, J.
Frank, G. Cruciani, S. Lorkowski, and N. K. Ozer. 2017. Vitamin ¨
E: emerging aspects and new directions. Free Radic. Biol. Med.
102:16–36.
Galobart, J., A. C. Barroeta, L. Cortinas, M. D. Baucells, and R.
Codony. 2002. Accumulation of alpha-tocopherol in eggs enriched with omega3 and omega6 polyunsaturated fatty acids. Poult. Sci.
81:1873–1876.
Garamszegi, L. Z., C. Biard, M. Eens, A. P. Møller, N. Saino, and
P. Surai. 2007. Maternal effects and the evolution of brain size in birds: overlooked developmental constraints. Neurosci. Biobehav.
Rev. 31:498–515.
Golzar Adabi, S. H., R. G. Cooper, M. A. Kamali, and A. Hajbabaei.
2011. The influence of inclusions of vitamin E and corn oil on semen traits of Japanese quail (Coturnix coturnix japonica). Anim.
Reprod. Sci. 123:119–125.
Hansen, H., T. Wang, D. Dolde, H. Xin, and K. Prusa. 2015. Supplementation of laying-hen feed with annatto tocotrienols and impact of α-tocopherol on tocotrienol transfer to egg yolk. J. Agric.
Food Chem. 63:2537–2544.
Hansen, J. M., and C. Harris. 2015. Glutathione during embryonic development. Biochim. Biophys. Acta. 1850:1527–1542.
Irandoust, H., and D. U. Ahn. 2015. Influence of soy oil source and dietary supplementation of vitamins E and C on the oxidation status of serum and egg yolk, and the lipid profile of egg yolk.
Poult. Sci. 94:2763–2771.
Islam, K. M., M. Khalil, K. M¨anner, J. Raila, H. Rawel, J. Zentek, and F. J. Schweigert. 2016. Effect of dietary α-tocopherol on the bioavailability of lutein in laying hen. J. Anim. Physiol. Anim.
Nutr. 100:868–875.
Jiang, W., L. Zhang, and A. Shan. 2013. The effect of vitamin E on laying performance and egg quality in laying hens fed corn dried distillers grains with solubles. Poult. Sci. 92:2956–2964.
Karadas, F., P. F. Surai, and N. H. Sparks. 2011. Changes in broiler chick tissue concentrations of lipid-soluble antioxidants immediately post-hatch. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160:68–71.
Karadas, F., A. P. Møller, and M. R. Karage¸cili. 2017. A comparison of fat-soluble antioxidants in wild and farm-reared chukar partridges (Alectoris chukar). Comp. Biochem. Physiol. A Mol.
Integr. Physiol. 208:89–94.
Karadas, F., P. Surai, E. Grammenidis, N. H. Sparks, and T.
Acamovic. 2006. Supplementation of the maternal diet with tomato powder and marigold extract: effects on the antioxidant system of the developing quail. Br. Poult. Sci. 47:200–
208.
Khan, R. U., S. Naz, Z. Nikousefat, V. Tufarelli, M. Javdani, N.
Rana, and V. Laudadio. 2011. Effect of vitamin E in heat-stressed poultry. World. Poult. Sci. J. 67:469–478.
Khan, W. A., M. Z. Khan, A. Khan, Z. Ul Hassan, and M. K.
Saleemi. 2014. Potential for amelioration of aflatoxin B1-induced immunotoxic effects in progeny of white leghorn breeder hens coexposed to vitamin E. J. Immunotoxicol. 11:116–125.
Lin, Y. F., S. J. Chang, J. R. Yang, Y. P. Lee, and A. L. Hsu. 2005a.
Effects of supplemental vitamin E during the mature period on the reproduction performance of Taiwan native chicken cockerels.
Br. Poult. Sci. 46:366–373.
Lin, Y. F., H. L. Tsai, Y. C. Lee, and S. J. Chang. 2005b. Maternal vitamin E supplementation affects the antioxidant capability and oxidative status of hatching chicks. J. Nutr. 135:2457–2461.
Long, J. A., and M. Kramer. 2003. Effect of vitamin E on lipid peroxidation and fertility after artificial insemination with liquidstored turkey semen. Poult. Sci. 82:1802–1807.
Lucas, A., J. Morales, and A. Velando. 2014. Differential effects of specific carotenoids on oxidative damage and immune response of gull chicks. J. Exp. Biol. 217:1253–1262.
Maldjian, A., S. Cerolini, P. F. Surai, and B. K. Speake. 1998. The effect of vitamin E, green tea extracts and catechin on the in vitro storage of turkey spermatozoa at room temperature. Poult.
Avian Biol. Rev. 9:143–151.
Matsumoto, Y., T. Terada, and Y. Tsutsumi. 1985. Glutamic oxaloacetic transaminase released from chicken spermatozoa during freeze-thaw procedures. Poult. Sci. 64:718–722.
Min, Y., T. Sun, Z. Niu, and F. Liu. 2016. Vitamin C and vitamin
E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Anim. Reprod. Sci. 171:1–6.
Min, Y. N., Z. Y. Niu, T. T. Sun, Z. P. Wang, P. X. Jiao, B. B. Zi,
P. P. Chen, D. L. Tian, and F. Z. Liu. 2018. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 97:1238–1244.
Monaghan, P., N. B. Metcalfe, and R. Torres. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12:75–92.
Naber, E. C. 1993. Modifying vitamin composition of eggs: a review.
J. Appl. Poult. Res. 2:385–393.
Panda, A. K., S. V. Rama Rao, M. V. L. N. Raju, and R. N. Chatterjee. 2007. Effect of vitamin E supplementation on performance, immune response and antioxidant status of white leghorn layers during heat stress. Indian J. Anim. Nutr. 23:226–230.
Panda, A. K., S. V. Ramarao, M. V. Raju, and R. N. Chatterjee.
2008. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of White Leghorn layers under tropical summer conditions.
Br. Poult. Sci. 49:592–599.
Panda, A. K., and G. Cherian. 2014. Role of vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 51:109–117.
Parolini, M., L. Khoriauli, C. D. Possenti, G. Colombo, M. Caprioli,
M. Santagostino, S. G. Nergadze, A. Milzani, E. Giulotto, and
N. Saino. 2017. Yolk vitamin E prevents oxidative damage in gull hatchlings. R. Soc. Open. Sci. 4:170098.
Rajashree, K., T. Muthukumar, and N. Karthikeyan. 2014. Comparative study of the effects of organic selenium on hen performance and productivity of broiler breeders. Br. Poult. Sci. 55:367–374.
Rengaraj, D., and Y. H. Hong. 2015. Effects of dietary vitamin E on fertility functions in poultry species. IJMS 16:9910–
9921.
Rimbach, G., J. Moehring, P. Huebbe, and J. K. Lodge. 2010. Generegulatory activity of α-tocopherol. Molecules 15:1746–1761.
Royle, N. J., P. F. Surai, and I. R. Hartley. 2003. The effect of variation in dietary intake on maternal deposition of antioxidants in zebra finch eggs. Funct. Ecol. 17:472–481.
Rui, B. R., F. Y. Shibuya, A. J. Kawaoku, J. D. A. Losano, D. S. R.
Angrimani, A. Dalmazzo, M. Nichi, and R. J. G. Pereira. 2017.
Impact of induced levels of specific free radicals and malondialdehyde on chicken semen quality and fertility. Theriogenology
90:11–19.
Safari Asl, R., F. Shariatmadari, M. Sharafi, M. A. Karimi Torshizi, and A. Shahverdi. 2018. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Ross breeder roosters fed a diet supplemented with a moderate ratio of n-3: n-6 fatty acids. Poult. Sci. doi:10.3382/ps/pey278
Siegel, P. B., S. E. Price, B. Meldrum, M. Picard, and P. A. Geraert.
2001. Performance of pureline broiler breeders fed two levels of vitamin E. Poult. Sci. 80:1258–1262.
Siegel, P. B., M. Blair, W. B. Gross, B. Meldrum, C. Larsen, K.
Boa-Amponsem, and D. A. Emmerson. 2006. Poult performance as influenced by age of dam, genetic line, and dietary vitamin E.
Poult. Sci. 85:939–942.
Speake, B. K., P. F. Surai, R. C. Noble, J. V. Beer, and N. Wood.
1999. Differences in egg lipid and antioxidant composition between wild and captive pheasants and geese. Comp. Biochem.
Physiol. B Biochem. Mol. Biol. 124:101–107.
Stock, M. K., K. K. Silvernail, and J. Metcalfe. 1990. Prenatal oxidative stress: I. malondialdehyde in hypoxic and hyperoxic chick embryos. Free Radic. Biol. Med. 8:313–318.
Sun, L. H., J. Q. Huang, J. Deng, and X. G. Lei. 2018. Avian selenogenome: response to dietary Se and vitamin E deficiency and supplementation. Poult. Sci. doi: 10.3382/ps/pey408
S¨under, A., and G. Flachowsky. 2001. Influence of high vitamin E dosages on retinol and carotinoid concentration in body tissues and eggs of laying hens. Arch. Tierernahr. 55:43–52.
Surai, A. P., P. F. Surai, W. Steinberg, W. G. Wakeman, B. K.
Speake, and N. H. Sparks. 2003. Effect of canthaxanthin content of the maternal diet on the antioxidant system of the developing chick. Br. Poult. Sci. 44:612–619.
Surai, P. F. 1981. Fat-soluble vitamins in turkey sperm. Pages 55–
56. Proceedings of 2nd Conference of Young Scientists, Zagorsk,
USSR.
Surai, P. F. 1982a. The effect of high doses of vitamin E in turkey male diet on the GOT and LDH activity in stored sperm. Nauˇcnotehniˇceskij bˆulleten´ – Ukrainskij nauˇcno-issledovatel´skij institut pticevodstva. 12:24–29.
Surai, P. F. 1982b. Vitamins A, E and C levels in the liver and testes of turkey males at the beginning and the end of the reproductive period. Nauˇcno-tehniˇceskij bˆulleten´ – Ukrainskij nauˇcnoissledovatel´skij institut pticevodstva. 13:30–33.
Surai, P. F. 1983a. Biochemical and functional changes in turkey tissues and sperm as a function of the levels of vitamins E and A in feed. Cand. Biol. Sci. (PhD) Thesis. Ukrainian Poultry Research
Institute, Borky, Ukraine.
Surai, P. F. 1983b. Stabilizing effect of vitamins A and E on turkey sperm membranes. Ptitsevodstvo (Kiev) 36:49–52.
Surai, P. F. 1984. Lipid peroxidation in turkey semen. Ptitsevodstvo (Kiev). 37:46–49.
Surai, P. F. 1988a. Alpha-tocopherol content in various organs and tissues of turkey males during reproductive period. Ptitsevodstvo (Kiev). 41:42–44.
Surai, P. F. 1988b. A protective effect of fat-soluble vitamins during turkey sperm cryopreservation. Abstr. Int. Conf. Achievements
Prospects Develop. Cryobiol. Cryomed., Kharkov, Ukraine 213–
214. (Abstr.)
Surai, P. F. 1989a. Relations between vitamin E concentration in poultry spermatozoa and some semen biochemical and physiological characteristics. Proc. 8th Int. Symp. Current Problems
Avian Genet., Smolenice, Czechoslovakia 171–173.
Surai, P. F. 1989b. Detergent treatment of poultry spermatozoa: release of some enzymes. Proc. 8th Int. Symp. Current Problems
Avian Genet, Smolenice, Czechoslovakia 174–175.
Surai, P. F. 1991. Nutritional and biochemical aspects of vitamins in poultry. DSc (Biol.) Thesis. Ukrainian Poultry Research Institute, Borky, Ukraine.
Surai, P. F. 1992. Vitamin E feeding of poultry males. Proc. 19th
World. Poult. Congr., Amsterdam, The Netherlands 1:575–577.
Surai, P. F. 1999a. Vitamin E in avian reproduction. Poult. Avian
Biol. Rev. 10:1–60.
Surai, P. F. 1999b. Tissue-specific changes in the activities of antioxidant enzymes during the development of the chicken embryo. Br.
Poult. Sci. 40:397–405.
Surai, P. F. 2000. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 41:235–243.
Surai, P. F. 2002. Natural Antioxidants in Avian Nutrition and Reproduction. Nottingham University Press, Nottingham, UK.
Surai, P. F. 2016. Antioxidant systems in poultry biology: superoxide dismutase. J. Anim. Res. Nutr. 1:8.
Surai, P. F. 2018. Selenium in Poultry Nutrition and Health. Wageningen Academic Publishers, Wageningen, The Netherlands.
Surai, P. F., and V. I. Fisinin. 2014. Antioxidant systems of the body: from vitamin E to polyphenols and beyond. Proc. 35th Western
Nutr. Conf., Edmonton, Canada 265–277.
Surai, P. F., and V. I. Fisinin. 2015. Antioxidant-prooxidant balance in the intestine: applications in chick placement and pig weaning.
J. Vet. Sci. Med. 3:1–16.
Surai, P. F., and V. I. Fisinin. 2016a. Vitagenes in poultry production: Part 1. technological and environmental stresses. World.
Poult. Sci. J. 72:721–734.
Surai, P. F., and V. I. Fisinin. 2016b. Vitagenes in poultry production: Part 2. nutritional and internal stresses. World. Poult. Sci.
J. 72:761–772.
Surai, P. F., and V. I. Fisinin. 2016c. Vitagenes in poultry production: Part 3. vitagene concept development. World. Poult. Sci. J.
72:793–804.
Surai, P. F., and V. I. Fisinin. 2016d. Natural antioxidants and stresses in poultry production: from vitamins to vitagenes. Proc.
25th Worlds Poult. Congr., Beijing, China 116–121.
Surai, P. F., and I. A. Ionov. 1981. Changes in GOT activity in turkey semen as a result of storage. Nauˇcno-tehniˇceskij bˆulleten´ –
Ukrainskij nauˇcno-issledovatel´skij institut pticevodstva. 11:24–
30.
Surai, P., and I. Ionov. 1992a. Vitamin E in fowl sperm. Proc. 12th
Int. Congr. Anim. Reprod., The Hague, The Netherlands 1:535–
537.
Surai, P., and I. Ionov. 1992b. Vitamin E in goose reproduction.
Proc. 9th Int. Symp. Waterfowl, Pisa, Italy 83–85.
Surai, P. F., and I. I. Kochish. 2017. Antioxidant systems and vitagenes in poultry biology: heat shock proteins. Pages 123-177 in
Heat Shock Proteins in Veterinary Medicine and Sciences. A. A.
A. Asea, and P. Kaur, eds. Springer, Cham, Switzerland.
Surai, P. F., and I. I. Kochish. 2018a. Nutritional modulation of antioxidant capacities in poultry: the case of selenium. Poult. Sci.
Surai, P. F., and I. I. Kochish. 2018b. Oxidative damage of biological molecules on animal metabolism and physiology. Proc. 2018
Anim. Nutr. Conf. Canada, Edmonton, Canada 234–251.
Surai, P. F., and N. H. C. Sparks. 2000. Tissue-specific fatty acid and α-tocopherol profiles in male chickens depending on dietary tuna oil and vitamin E provision. Poult. Sci. 79:1132–1142.
Surai, P. F., and N. H. Sparks. 2001. Comparative evaluation of the effect of two maternal diets on fatty acids, vitamin E and carotenoids in the chick embryo. Br. Poult. Sci. 42:252–
259.
Surai, P. F, and B. K. Speake. 1998. Selective excretion of yolkderived tocotrienols into the bile of the chick embryo. Comp.
Biochem. Physiol. B Biochem. Mol. Biol. 121:393–396.
Surai, P., R. Noble, and B. Speake. 1996. Tissue-specific differences in antioxidant distribution and susceptibility to lipid peroxidation during development of the chick embryo. Biochim. Biophys. Acta.
1304:1–10.
Surai, P. F., E. Kutz, G. J. Wishart, R. C. Noble, and B. K. Speake.
1997a. The relationship between the dietary provision of αtocopherol and the concentration of this vitamin in the semen of chicken: effects on lipid composition and susceptibility to peroxidation. Reproduction 110:47–51.
Surai, P., T. Gaal, R. Noble, and B. Speake. 1997b. The relationship between α-tocopherol content of the yolk and its accumulation in the tissues of the newly hatched chick. J. Sci. Food Agric.
75:212–216.
Surai, P. F., E. Blesbois, I. Grasseau, T. Ghalah, J.-P. Brillard, G.
Wishart, S. Cerolini, and N. H. C. Sparks. 1998a. Fatty acid composition, glutathione peroxidase and superoxide dismutase activity and total antioxidant activity of avian semen. Comp. Biochem.
Physiol. B Biochem. Mol. Biol. 120B:527–533.
Surai, P. F., S. Cerolini, G. J. Wishart, B. K. Speake, R. C. Noble, and N. H. C. Sparks. 1998b. Lipid and antioxidant composition of chicken semen and its susceptibility to peroxidation. Poult. Avian
Biol. Rev. 9:11–23.
Surai, P., I. Ionov, E. Kuchmistova, R. C. Noble, and B. K. Speake.
1998c. The relationship between the levels of α-tocopherol and carotenoids in the maternal feed, yolk and neonatal tissues: comparison between the chicken, turkey, duck and goose. J. Sci. Food
Agric. 76:593–598.
Surai, P. F., I. A. Kostjuk, G. Wishart, A. MacPherson, B. Speake,
R. C. Noble, I. A. Ionov, and E. Kutz. 1998d. Effect of vitamin E and selenium supplementation of cockerel diets on glutathione peroxidase activity and lipid peroxidation susceptibility in sperm, testes, and liver. Biol. Trace Elem. Res. 64:119–
132.
Surai, P. F., R. C. Noble, and B. K. Speake. 1999a. Relationship between vitamin E content and susceptibility to lipid peroxidation in tissues of the newly hatched chick. Br. Poult. Sci. 40:406–
410.
Surai, P. F., B. K. Speake, R. C. Noble, and N. H. C. Sparks.
1999b. Tissue-specific antioxidant profiles and susceptibility to lipid peroxidation of the newly hatched chick. Biol. Trace Elem.
Res. 68:63–78.
Surai, P. F., N. H. Sparks, and R. C. Noble. 1999c. Antioxidant systems of the avian embryo: tissue-specific accumulation and distribution of vitamin E in the turkey embryo during development.
Br. Poult. Sci. 40:458–466.
Surai, P. F., R. C. Noble, N. H. C. Sparks, and B. K. Speake. 2000a.
Effect of long-term supplementation with arachidonic or docosahexaenoic acids on sperm production in the broiler chicken. J.
Reprod. Fertil. 120:257–264.
Surai, P. F., J.-P. Brillard, B. K. Speake, E. Blesbois, F. Seigneurin, and N. H. C. Sparks. 2000b. Phospholipid fatty acid composition, vitamin E content and susceptibility to lipid peroxidation of duck spermatozoa. Theriogenology. 53:1025–1039.
Surai, P. F., A. MacPherson, B. K. Speake, and N. H. C. Sparks.
2000c. Designer egg evaluation in a controlled trial. Eur. J. Clin.
Nutr. 54:298–305.
Surai, P. F., G. R. Bortolotti, A. Fidgett, J. Blount, and B. K.
Speake. 2001a. Effects of piscivory on the fatty acid profiles and antioxidants of avian yolk: studies on eggs of the gannet, skua, pelican and cormorant. J. Zool. 255:305–312.
Surai, P. F., N. Fujihara, B. K. Speake, J. P. Brillard, G. J. Wishart, and N. H. C. Sparks. 2001b. Polyunsaturated fatty acids, lipid peroxidation and antioxidant protection in avian semen. AsianAustralas. J. Anim. Sci. 14:1024–1050.
Surai, P. F., V. I. Fisinin, and F. Karadas. 2016. Antioxidant systems in chick embryo development. Part 1. vitamin E, carotenoids and selenium. Anim. Nutr. 2:1–11.
Surai, P. F., I. I. Kochish, and V. I. Fisinin. 2017a. Antioxidant systems in poultry biology: nutritional modulation of vitagenes.
Eur. Poult. Sci. 81. doi:10.1399/eps.2017.214
Surai, P. F., I. I. Kochish, D. K. Griffin, I. N. Nikonov, and M. N.
Romanov. 2017b. Microbiome and antioxidant system of the gut in chicken: food for thoughts. Insights Nutr. Metab. 1:34. (Abstr.)
Surai, P., I. Kochish, and V. Fisinin. 2018c. Glutathione peroxidases in poultry biology: Part 1. classification and mechanisms of action. World. Poult. Sci. J. 74:185–198.
Surai, P., I. Kochish, and V. Fisinin. 2018d. Glutathione peroxidases in poultry biology: Part 2. modulation of enzymatic activities.
World. Poult. Sci. J. 74:239–250.
Tasinato, A., D. Boscoboinik, G. M. Bartoli, P. Maroni, and A.
Azzi. 1995. d-α-Tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc. Natl. Acad. Sci. 92:12190–12194.
Timme-Laragy, A. R., M. E. Hahn, J. M. Hansen, A. Rastogi, and
M. A. Roy. 2018. Redox stress and signaling during vertebrate embryonic development: regulation and responses. Semin. Cell
Dev. Biol. 80:17–28.
Tinsley, I. J., G. H. Arscott, and R. R. Lowry. 1971. Fertility and testicular fatty acid composition in the chicken as influenced by vitamin E and ethoxyquin. Lipids 6:657–660.
Tsai, H. L., S. K. Chang, Y. F. Lin, and S. J. Chang. 2008. Beneficial effects of maternal vitamin E supplementation on the antioxidant system of the neonate chick brain. Asian-Australas. J. Anim. Sci.
21:225–231.
Walde, C. M., A. M. Drotleff, and W. Ternes. 2014. Comparison of dietary tocotrienols from barley and palm oils in hen’s egg yolk: transfer efficiency, influence of emulsification, and effect on egg cholesterol. J. Sci. Food Agric. 94:810–818.
Wales, R. G., I. G. White, and D. R. Lammond. 1959. The spermicidal activity of hydrogen peroxide in vitro and in vivo. J.
Endocrinol. 18:236–244.
Watson, H., P. Salm´on, and C. Isaksson. 2018. Maternally derived yolk antioxidants buffer the developing avian embryo against oxidative stress induced by hyperoxia. J. Exp. Biol. 221. pii: jeb179465.
Yigit, A. A., A. K. Panda, and G. Cherian. 2014. The avian embryo and its antioxidant defence system. Worlds Poult. Sci. J. 70:563–
574.
Zaniboni, L., and S. Cerolini. 2009. Liquid storage of turkey semen: changes in quality parameters, lipid composition and susceptibility to induced in vitro peroxidation in control, n-3 fatty acids and alpha-tocopherol rich spermatozoa. Anim. Reprod. Sci.
112:51–65.
Zaniboni, L., R. Rizzi, and S. Cerolini. 2006. Combined effect of DHA and alpha-tocopherol enrichment on sperm quality and fertility in the turkey. Theriogenology 65:1813–1827.
Zdu´nczyk, Z., A. Dra˙zbo, J. Jankowski, J. Ju´skiewicz, A. Czech, and
Z. Antoszkiewicz. 2013. The effect of different dietary levels of vitamin E and selenium on antioxidant status and immunological markers in serum of laying hens. Polish J. Vet. Sci. 16:333–339.
Zingg, J. M. 2018. Vitamin E: Regulatory Role on Signal
Transduction. IUBMB Life.








.jpg&w=3840&q=75)