Modulating Laying Hens Productivity and Immune Performance in Response to Oxidative Stress Induced by E. coli Challenge Using Dietary Propolis Supplementation
1 Department of Animal and Fish Production, College of Agricultural and Food Sciences, King Faisal University, P.O. Box 420, Al-Ahsa 31982, Saudi Arabia; 2 Department of Animal Production, Faculty of Agriculture, Cairo University, Gamma St., Giza, Cairo P.O. Box 12613, Egypt; 3 Department of Agricultural Biotechnology, College of Agricultural and Food Sciences, King Faisal University, P.O. Box 420, Al-Ahsa 31982, Saudi Arabia; 4 Department of Biochemistry, Faculty of Agriculture Cairo University, Gamma St., Giza, Cairo P.O. Box 12613, Egypt; 5 Department of Poultry Breeding, Animal Production Research Institute, Agricultural Research Center, Dokki, Giza P.O. Box 12611, Egypt; 6 Department of Animal Production, National Research Center, El Buhouth St., Dokki, Giza, Cairo P.O. Box 12622, Egypt.
Propolis (PR) is a resin product of bee colonies that has rich bioactive antioxidant and bactericidal compounds. Endotoxin, a byproduct of bacterial growth, is reported to cause progressive induction of endogenous oxidative stress and has negative impacts on individual health and wellbeing. Hereby, we investigated the ability of PR to alleviate the oxidative stress and immunosuppression imposed by avian pathogenic Escherichia coli using laying hen as a based model. In this study, PR was dietary supplemented to hens for 4 weeks at a concentration of 0.1%. At the beginning of the 4th week of the experiment, hens from control and PR treatment were injected with E. coli (O157:H7; 107 colonies/hen) or saline. The results showed significant (p < 0.05) negative impact of E. coli challenge on antioxidant status, immune response and productive performance. PR supplementation reduced (p < 0.05) inflammation markers levels (tumor necrosis factor α (TNFα) and interleukin 1β (IL-1β)) and plasma corticosterone concentration. The antioxidant status was ameliorated with dietary PR supplementation to challenged hens, showing significant (p < 0.05) reduction in malondialdehyde (MDA) levels and increasing total antioxidant capacity (TAC) concentrations. Cell mediated, as well as, humeral immune response improved significantly (p < 0.05) with dietary PR verified by the enhancement of T- and B-lymphocyte proliferation and the positive respond to phytohemagglutinin (PHA). Leucocyte cells viability increased significantly and the apoptotic factor forkhead box O3 (Foxo3) was reduced with PR supplementation. The current study revealed that dietary PR supplementation can effectively be used as an organic feed additive to overcome the endogenous oxidative stress induced by endotoxins challenge.
Keywords: propolis; E. coli; inflammation markers; lymphocyte proliferation; leucocyte cell viability; Foxo3; laying hen.
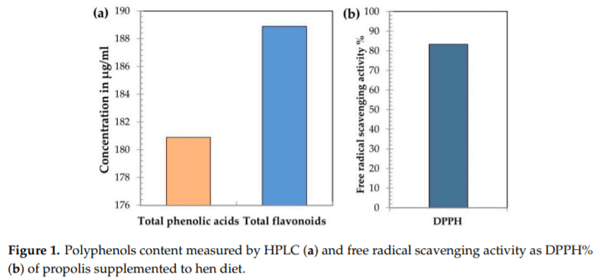
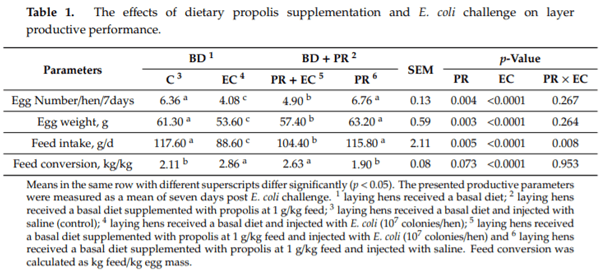
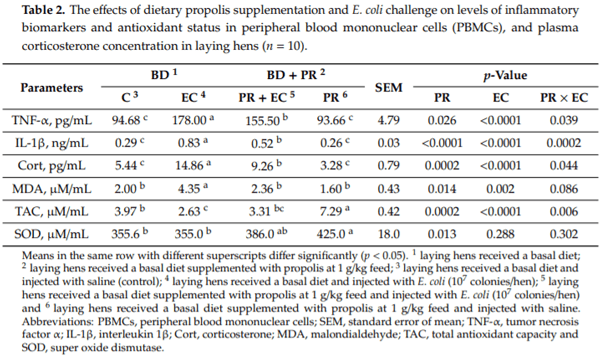
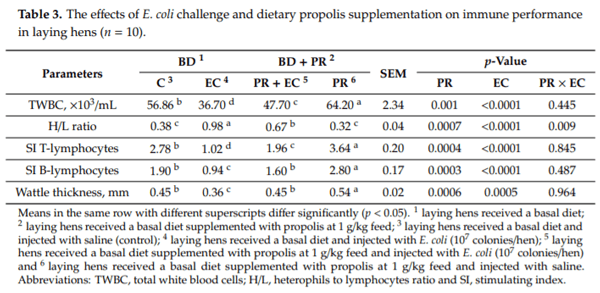
- Saiyasit, N.; Chunchai, T.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Chronic high-fat diet consumption induces an alteration in plasma/brain neurotensin signaling, metabolic disturbance, systemic inflammation/oxidative stress, brain apoptosis, and dendritic spine loss. Neuropeptides 2020, 82, 102047. [CrossRef] [PubMed]
- Singh, A.; Tripathi, P.; Yadawa, A.K.; Singh, S. Promising Polyphenols in Parkinson’s Disease Therapeutics. Neurochem. Res. 2020, 45, 1731–1745. [CrossRef] [PubMed]
- Son, E.S.; Park, J.-W.; Kim, Y.J.; Jeong, S.H.; Hong, J.H.; Kim, S.-H.; Kyung, S.Y. E ects of antioxidants on oxidative stress and inflammatory responses of human bronchial epithelial cells exposed to particulate matter and cigarette smoke extract. Toxicol. In Vitro 2020, 67, 104883. [CrossRef] [PubMed]
- Petushkova, A.I.; Zamyatnin, J.A.A. Redox-Mediated Post-Translational Modifications of Proteolytic Enzymes and Their Role in Protease Functioning. Biomolecules 2020, 10, 650. [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2014, 74, 5–17. [CrossRef]
- Enany, M.E.; Algammal, A.M.; Nasef, S.A.; Abo-Eillil, S.A.M.; Bin-Jumah, M.N.; Taha, A.E.; Allam, A.A. The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express 2019, 9, 1–9. [CrossRef] [PubMed]
- Al Azad, M.A.R.; Rahman, M.; Amin, R.; Begum, M.I.A.; Fries, R.; Husna, A.; Khairalla, A.S.; Badruzzaman, A.; El Zowalaty, M.E.; Na Lampang, K.; et al. Susceptibility and Multidrug Resistance Patterns of Escherichia coli Isolated from Cloacal Swabs of Live Broiler Chickens in Bangladesh. Pathogens 2019, 8, 118. [CrossRef]
- Muloi, D.; Kiiru, J.; Ward, M.J.; Hassell, J.M.; Bettridge, J.M.; Robinson, T.P.; Van Bunnik, B.A.; Chase-Topping, M.; Robertson, G.; Pedersen, A.B.; et al. Epidemiology of antimicrobial-resistant Escherichia coli carriage in sympatric humans and livestock in a rapidly urbanizing city. Int. J. Antimicrob. Agents 2019, 54, 531–537. [CrossRef]
- Zbikowska,˙ K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [CrossRef]
- Franklin, N.; Hope, K.; Glasgow, K.; Glass, K. Describing the Epidemiology of Foodborne Outbreaks in New South Wales from 2000 to 2017. Foodborne Pathog. Dis. 2020. [CrossRef] [PubMed]
- Ibrahim, R.A.; Cryer, T.L.; Lafi, S.; Abu-Basha, E.A.; Good, L.; Tarazi, Y.H. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Veter. Res. 2019, 15, 159. [CrossRef]
- Yan, W.; Bai, R.; Wang, S.; Tian, X.; Li, Y.; Wang, S.; Yang, F.; Xiao, Y.; Lu, X.; Zhao, F. Antibiotic resistance genes are increased by combined exposure to sulfamethoxazole and naproxen but relieved by low-salinity. Environ. Int. 2020, 139, 105742. [CrossRef] [PubMed]
- Han, T.; Zhang, Q.; Liu, N.; Wang, J.; Li, Y.; Huang, X.; Liu, J.; Wang, J.; Qu, Z.; Qi, K. Changes in antibiotic resistance of Escherichia coli during the broiler feeding cycle. Poult. Sci. 2020. [CrossRef]
- Kumari, M.; Gupta, R.P.; Lather, D.; Bagri, P. Ameliorating e ect of Withania somnifera root extract in Escherichia coli–infected broilers. Poult. Sci. 2020, 99, 1875–1887. [CrossRef] [PubMed]
- Daneshmand, A.; Kermanshahi, H.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M. Antimicrobial peptide, cLF36, a ects performance and intestinal morphology, microflora, junctional proteins, and immune cells in broilers challenged with E. coli. Sci. Rep. 2019, 9, 14176–14179. [CrossRef]
- Ramadan, H.; Jackson, C.R.; Frye, J.G.; Hiott, L.M.; Samir, M.; Awad, A.; Woodley, T.A. Antimicrobial Resistance, Genetic Diversity and Multilocus Sequence Typing of Escherichia coli from Humans, Retail Chicken and Ground Beef in Egypt. Pathogens 2020, 9, 357. [CrossRef]
- Hazrati, S.; Rezaeipour, V.; Asadzadeh, S. E ects of phytogenic feed additives, probiotic and mannan-oligosaccharides on performance, blood metabolites, meat quality, intestinal morphology, and microbial population of Japanese quail. Br. Poult. Sci. 2019, 61, 132–139. [CrossRef]
- Dong, Y.; Li, R.; Liu, Y.; Ma, L.; Zha, J.; Qiao, X.; Chai, T.; Wu, B. Benefit of Dietary Supplementation with Bacillus subtilis BYS2 on Growth Performance, Immune Response, and Disease Resistance of Broilers. Probiot. Antimicrob. Proteins 2020. [CrossRef]
- Makled, M.N.; Abouelezz, K.; Gad-Elkareem, A.E.G.; Sayed, A.M. Comparative influence of dietary probiotic, yoghurt, and sodium butyrate on growth performance, intestinal microbiota, blood hematology, and immune response of meat-type chickens. Trop. Anim. Health Prod. 2019, 51, 2333–2342. [CrossRef]
- Zhan, H.; Dong, X.; Li, L.; Zheng, Y.; Gong, Y.; Zou, X. E ects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019, 98, 896–903. [CrossRef]
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis Counteracts Some Threats to Honey Bee Health. Insects 2017, 8, 46. [CrossRef]
- Rivero-Cruz, J.F.; Granados-Pineda, J.; Pedraza-Chaverri, J.; Rojas, J.M.P.; Passari, A.K.; Díaz-Ruiz, G.; Rivero-Cruz, B.E. Phytochemical Constituents, Antioxidant, Cytotoxic, and Antimicrobial Activities of the Ethanolic Extract of Mexican Brown Propolis. Antioxidants 2020, 9, 70. [CrossRef] [PubMed]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Di erent Forms and in Di erent Solvents Useful for Finished Products. Foods 2018, 7, 41. [CrossRef]
- Wo´zniak, M.; Mrówczynska,´ L.; Wa´skiewicz, A.; Rogozinski,´ T.; Ratajczak, I. Phenolic Profile and Antioxidant Activity of Propolis Extracts from Poland. Nat. Prod. Commun. 2019, 14. [CrossRef]
- Osés, S.; Marcos, P.; Azofra, P.; De Pablo, A.; Fernández-Muíño, M.A.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Di erent Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [CrossRef]
- Kuo, Y.-H.; Chiang, H.-L.; Wu, P.-Y.; Chu, Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protection against Ultraviolet A-Induced Skin Apoptosis and Carcinogenesis through the Oxidative Stress Reduction E ects of N-(4-bromophenethyl) Ca eamide, A Propolis Derivative. Antioxidants 2020, 9, 335. [CrossRef]
- Ramanauskiene,˙ K.; Inkenien˙e,˙ A.M.; Petrikaite,˙ V.; Briedis, V. Total Phenolic Content and Antimicrobial Activity of Di erent Lithuanian Propolis Solutions. Evid. Based Complement. Altern. Med. 2013, 2013, 1–5. [CrossRef]
- Park, E.-H.; Kim, S.-H.; Park, S.-S. Anti-inflammatory activity of propolis. Arch. Pharm. Res. 1996, 19, 337–341. [CrossRef]
- Campos, J.F.; Dos Santos, E.L.; da Rocha, P.D.S.; Damião, M.J.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; Estevinho, L.M.; Souza, K.D.P.; Dos Santos, E.L. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca fiebrigi (Jataí). Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. [CrossRef]
- Abass, A.O.; Kamel, N.; Khalifa, W.; Gouda, G.F.; El-Manylawi, M.A.F.; Mehaisen, G.M.K.; Mashaly, M.M. Propolis supplementation attenuates the negative e ects of oxidative stress induced by paraquat injection on productive performance and immune function in turkey poults. Poult. Sci. 2017, 96, 4419–4429. [CrossRef] [PubMed]
- Prakatur, I.; Miškulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miškulin, M.; Domacinovic, M. Intestinal Morphology in Broiler Chickens Supplemented with Propolis and Bee Pollen. Animals 2019, 9, 301. [CrossRef] [PubMed]
- Mehaisen, G.M.K.; Ibrahim, R.M.; Desoky, A.A.; Safaa, H.; El-Sayed, O.A.; Abass, A.O. The importance of propolis in alleviating the negative physiological effects of heat stress in quail chicks. PLoS ONE 2017, 12, e0186907. [CrossRef] [PubMed]
- Mehaisen, G.M.K.; Desoky, A.A.; Sakr, O.G.; Sallam, W.; Abass, A.O. Propolis alleviates the negative e ects of heat stress on egg production, egg quality, physiological and immunological aspects of laying Japanese quail. PLoS ONE 2019, 14, e0214839. [CrossRef] [PubMed]
- AlQarni, A.M.; Niwasabutra, K.; Sahlan, M.; Fearnley, H.; Ferro, V.; Watson, D.G. Propolis Exerts an Anti-Inflammatory E ect on PMA-Di erentiated THP-1 Cells via Inhibition of Purine Nucleoside Phosphorylase. Metabolites 2019, 9, 75. [CrossRef]
- Mehaisen, G.M.K.; Eshak, M.G.; Elkaiaty, A.M.; Atta, A.M.; Mashaly, M.M.; Abass, A.O. Comprehensive growth performance, immune function, plasma biochemistry, gene expressions and cell death morphology responses to a daily corticosterone injection course in broiler chickens. PLoS ONE 2017, 12, e0172684. [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [CrossRef]
- Oktay, M.; Gülçin, I.; Küfrevioglu,˘ Ö.I. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT 2003, 36, 263–271. [CrossRef]
- Edelman, A.S.; Sánchez, P.L.; Robinson, M.E.; Hochwald, G.M.; Thorbecke, G. Primary and Secondary Wattle Swelling Response to Phytohemagglutinin as a Measure of Immunocompetence in Chickens. Avian Dis. 1986, 30, 105. [CrossRef]
- Gehad, A.E.; Mehaisen, G.M.K.; Abbas, A.O.; Mashaly, M.M. The Role of Light Program and Melatonin on Alleviation of Inflammation Induced by Lipopolysaccharide Injection in Broiler Chickens. Int. J. Poult. Sci. 2008, 7, 193–201. [CrossRef]
- Campbell, T.W. Hematology. In Avian Medicine: Principles and Application; Ritchie, B.W., Harrison, G.J., Harrison, L.R., Eds.; Winger’s Publishing Inc.: Lake Worth, FL, USA, 1994; pp. 176–198.
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; p. 584. [CrossRef]
- Reinhold, D.; Bank, U.; Bühling, F.; Neubert, K.; Mattern, T.; Ulmer, A.J.; Flad, H.-D.; Ansorge, S. Dipeptidyl Peptidase IV (CD26) on Human Lymphocytes. Synthetic Inhibitors of and Antibodies against Dipeptidyl Peptidase IV Suppress the Proliferation of Pokeweed Mitogen-Stimulated Peripheral Blood Mononuclear Cells, and IL-2 and IL-6 Production. Immunobiology 1993, 188, 403–414. [CrossRef]
- SAS Institute Inc. SAS/STATR 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004.
- Kirikae, T.; Kirikae, F.; Saito, S.; Tominaga, K.; Tamura, H.; Uemura, Y.; Yokochi, T.; Nakano, M. Biological Characterization of Endotoxins Released from Antibiotic-Treated Pseudomonas aeruginosa and Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 1015–1021. [CrossRef] [PubMed]
- Mahmoud, K.Z.; Edens, F. Influence of organic selenium on hsp70 response of heat-stressed and enteropathogenic Escherichia coli-challenged broiler chickens (Gallus gallus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 141, 69–75. [CrossRef] [PubMed]
- Chaudhari, A.A.; Kariyawasam, S. An Experimental Infection Model for Escherichia coli Egg Peritonitis in Layer Chickens. Avian Dis. 2014, 58, 25–33. [CrossRef]
- Surai, P.; Kochish, I.I.; Fisinin, V.I.; Kidd, M. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [CrossRef]
- Da Rosa, G.; Alba, D.F.; Silva, A.D.; Gris, A.; Mendes, R.E.; Mostardeiro, V.B.; Lopes, T.F.; Schetinger, M.R.C.; Stefani, L.M.; Lopes, M.T.; et al. Impact of Escherichia coli infection in broiler breeder chicks: The e ect of oxidative stress on weight gain. Microb. Pathog. 2019, 139, 103861. [CrossRef]
- Asgharpour, F.; Moghadamnia, A.A.; Motallebnejad, M.; Nouri, H.R. Propolis attenuates lipopolysaccharide-induced inflammatory responses through intracellular ROS and NO levels along with downregulation of IL-1 and IL-6 expressions in murine RAW 264.7 macrophages. J. Food Biochem. 2019, 43, e12926. [CrossRef]
- Stepanovi´c, S.; Anti´c, N.; Daki´c, I.; Švabi´c-Vlahovi´c, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [CrossRef]
- Abdel-Kareem, A.A.A.; El-Sheikh, T.M. Impact of supplementing diets with propolis on productive performance, egg quality traits and some haematological variables of laying hens. J. Anim. Physiol. Anim. Nutr. 2015, 101, 441–448. [CrossRef]
- Seven, P.T. The E ects of Dietary Turkish Propolis and Vitamin C on Performance, Digestibility, Egg Production and Egg Quality in Laying Hens under Di erent Environmental Temperatures. Asian Australas. J. Anim. Sci. 2008, 21, 1164–1170. [CrossRef]
- Kaˇcániová, M.; Rovná, K.; Arpášová, H.; Cubon,ˇˇ J.; Hleba, L.; Pochop, J.; Kunová, S.; Hašˇcík, P. In vitroandIn vivoantimicrobial activity of propolis on the microbiota from gastrointestinal tract of chickens. J. Environ. Sci. Health Part A 2012, 47, 1665–1671. [CrossRef]
- Mahmoud, U.T.; Cheng, H.; Applegate, T. Functions of propolis as a natural feed additive in poultry. Worlds Poult. Sci. J. 2016, 72, 37–48. [CrossRef]
- The Effect of Diet Propolis Supplementation on Ross Broiler Chicks Performance. Int. J. Poult. Sci. 2006, 5, 84–88. [CrossRef]
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [CrossRef]
- Mehaisen, G.M.K.; Eshak, M.G.; El Sabry, M.; Abass, A.O. Expression of Inflammatory and Cell Death Program Genes and Comet DNA Damage Assay Induced by Escherichia coli in Layer Hens. PLoS ONE 2016, 11, e0158314. [CrossRef]
- Turnbull, A.V.; Rivier, C. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [CrossRef]
- Løtvedt, P.; Fallahshahroudi, A.; Bektic, L.; Altimiras, J.; Jensen, P. Chicken domestication changes expression of stress-related genes in brain, pituitary and adrenals. Neurobiol. Stress 2017, 7, 113–121. [CrossRef]
- Peng, L.; Matthijs, M.G.; Haagsman, H.P.; Veldhuizen, E.J. Avian pathogenic Escherichia coli-induced activation of chicken macrophage HD11 cells. Dev. Comp. Immunol. 2018, 87, 75–83. [CrossRef]
- Mol, N.; Peng, L.; Esnault, E.; Quéré, P.; Haagsman, H.P.; Veldhuizen, E.J. Avian pathogenic Escherichia coli infection of a chicken lung epithelial cell line. Veter. Immunol. Immunopathol. 2019, 210, 55–59. [CrossRef]
- Kittana, H.; Neto, J.C.G.; Heck, K.; Geis, A.L.; Muñoz, R.R.S.; Cody, L.A.; Schmaltz, R.J.; Bindels, L.B.; Sinha, R.; Hostetter, J.M.; et al. Commensal Escherichia coli Strains Can Promote Intestinal Inflammation via Di erential Interleukin-6 Production. Front. Immunol. 2018, 9, 2318. [CrossRef] [PubMed]
- Ansorge, S.; Reinhold, D.; Lendeckel, U. Propolis and Some of its Constituents Down-Regulate DNA Synthesis and Inflammatory Cytokine Production but Induce TGF- 1 Production of Human Immune Cells. Z. Nat. C 2003, 58, 580–589. [CrossRef] [PubMed]
- Seven, P.T.; Yilmaz, S.; Seven, I.; Kelestemur, G.T. The E ects of Propolis in Animals Exposed Oxidative Stress. In Oxidative Stress—Environmental Induction and Dietary Antioxidants; Lushchak, V.I., Ed.; InTech: London, UK, 2012; pp. 267–288. [CrossRef]
- Ayala, A.; Muñoz, M.F.; Arguelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [CrossRef]
- Halawa, A.A.; El-Adl, M.A.; Hamed, M.F.; Balboula, A.Z.; Elmetwally, M. Lipopolysaccharide Prompts Oxidative Stress and Apoptosis in Rats’ Testicular Tissue. J. Veter. Health 2018, 1, 20–31. [CrossRef]
- Yangi, B.; Ustuner, M.C.; Dinçer, M.; Özbayer, C.; Tekin, N.; Üstüner, D.; Colak, E.; Kolac, U.K.; Entok, E. Propolis Protects Endotoxin Induced Acute Lung and Liver Inflammation Through Attenuating Inflammatory Responses and Oxidative Stress. J. Med. Food 2018, 21, 1096–1105. [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 1–29. [CrossRef]
- Wang, S.; Peng, Q.; Jia, H.M.; Zeng, X.F.; Zhu, J.L.; Hou, C.L.; Liu, X.T.; Yang, F.J.; Qiao, S.Y. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [CrossRef]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.-L.; Conlon, M.A.; Topping, D.L. Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating AMPK and ERK signaling. Nutrients 2016, 8, 272. [CrossRef]
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.-Q.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393. [CrossRef]
- Marshall, J.C.; Christou, N.V.; Meakins, J.L. Small-bowel bacterial overgrowth and systemic immunosuppression in experimental peritonitis. Surgery 1988, 104, 404–411.
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [CrossRef] [PubMed]
- Larbi, A.; Kempf, J.; Pawelec, G. Oxidative stress modulation and T cell activation. Exp. Gerontol. 2007, 42, 852–858. [CrossRef] [PubMed]
- Moro-García, M.A.; Mayo, J.C.; Sainz, R.M.; Alonso-Arias, R. Influence of Inflammation in the Process of T Lymphocyte Di erentiation: Proliferative, Metabolic, and Oxidative Changes. Front. Immunol. 2018, 9. [CrossRef]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [CrossRef]
- Tohyama, Y.; Takano, T.; Yamamura, H. B cell responses to oxidative stress. Curr. Pharm. Des. 2004, 10, 835–839. [CrossRef]
- Wagner, A.; Garner-Spitzer, E.; Jasinska,´ J.; Kollaritsch, H.; Stiasny, K.; Kundi, M.; Wiedermann, U. Age-related di erences in humoral and cellular immune responses after primary immunisation: Indications for stratified vaccination schedules. Sci. Rep. 2018, 8, 9825. [CrossRef]
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.L.G.; Liberal, J.; Cruz, M.T.; Lopes, M.; Batista, M.T.; Sforcin, J.M. Propolis and its constituent ca eic acid suppress LPS-stimulated pro-inflammatory response by blocking NF- B and MAPK activation in macrophages. J. Ethnopharmacol. 2013, 149, 84–92. [CrossRef]
- Stefanetti, R.J.; Voisin, S.; Russell, A.P.; Lamon, S. Recent advances in understanding the role of FOXO3. F1000Research 2018, 7, 1372. [CrossRef]
- Cui, C.; Han, S.; Yin, H.; Luo, B.; Shen, X.; Yang, F.; Liu, Z.; Zhu, Q.; Li, D.; Wang, Y. FOXO3 Is Expressed in Ovarian Tissues and Acts as an Apoptosis Initiator in Granulosa Cells of Chickens. BioMed Res. Int. 2019, 2019, 6902906. [CrossRef]
- Wang, P.; Geng, J.; Gao, J.; Zhao, H.; Li, J.; Shi, Y.; Yang, B.; Xiao, C.; Linghu, Y.; Sun, X.; et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 1–16. [CrossRef]
- Peng, S.L. Foxo in the immune system. Oncogene 2008, 27, 2337–2344. [CrossRef] [PubMed]
- Cabrera-Ortega, A.; Feinberg, D.; Liang, Y.; Rossa, J.C.; Graves, D.T. The Role of Forkhead Box 1 (FOXO1) in the Immune System: Dendritic Cells, T Cells, B Cells, and Hematopoietic Stem Cells. Crit. Rev. Immunol. 2017, 37, 1–13. [CrossRef]
- Bi, Y.; Xu, L.; Qiu, L.; Wang, S.; Liu, X.; Zhang, Y.; Chen, Y.; Xu, Q.; Chang, G.; Chen, G.; et al. Reticuloendotheliosis Virus Inhibits the Immune Response Acting on Lymphocytes from Peripheral Blood of Chicken. Front. Physiol. 2018, 9, 1–9. [CrossRef]
- Du, W.W.; Fang, L.; Yang, W.; Wu, N.; Awan, F.M.; Yang, Z.; Yang, B.B. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Di er. 2016, 24, 357–370. [CrossRef]








.jpg&w=3840&q=75)