intestinal surface microenvironment in poultry
Physiological dynamics at the intestinal brush border
Published: September 18, 2007
By: ZEHAVA UNI - Hebrew University (Courtesy of Alltech Inc.)
The following paper examines some of the processes that occur in the brush border region, among which are digestion and absorption capability, mucin coating and microflora colonization.
Introduction
Running from the stomach to the colon, the small intestine consists of three portions: duodenum, jejunum and ileum, respectively, which augment digestion/absorption in four ways.
First, these segments elongate the digestive tract. Secondly, on the internal aspect of the tube, folds in the mucosa layer increase the mucosal surface area nearly 3-fold. A third and more effective augmentation is achieved in the form of enormous numbers of villi that increase the luminal surface area about 8-fold (Figure 1A).
Each villus is covered by an epithelial layer one cell thick composed mostly of enterocyte cells. Granger and Baker (1950) were the first to show, by means of electron microscopy, that the luminal cell border is a ‘brush border’ comprised of an additional set of projections, the microvilli (Figure 1B).
Their presence on the membrane of the enterocytes increases the luminal surface of the small intestine by a factor of about 20.
Interspersed between the enterocyte cells are the goblet cells, which occur in increasing frequency towards the distal ileum. The apical two-thirds of these cells are filled with mucin secretory granules. Once secreted, the mucus forms a coating over the microvilli.
The digestive tract is colonized immediately after hatch (or birth) by a variety of microorganisms. Within the gastrointestinal population there are about 400 different types of bacteria in equilibrium with each other and with the host animal. Constant selective pressures produce microflora characteristic of each host species.
Part of this selection is chemical, due to inhibitory agents (e.g., volatile fatty acids (VFA), hydrogen sulphide, bile acids, lysozyme and immunoglobulins). When bacteria overcome these inhibitory effects they must then contend with the constant flushing effect of the peristaltic movement of food from the anterior regions of the gut. Bacteria remain in the intestine by attachment either to the mucus layer and/or to the brush border region of enterocytes lining the intestine (Figure 1C).

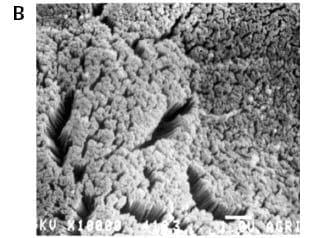

Figure 1.Three views of chick intestinal villi (scanning electron micrographs). A) Villi shape in the jejunum of 4-day-old chicks (X 430). B) The absorptive surface of the intestinal epithelial cells is lined with 2-4 μmlong microvilli (X 10,000). The microvillar membrane contains receptors, ion channels, pumps and transport systems. This region has importance in digestion and absorption processes, bacterial colonization and defense mechanisms. C) Bacterial colonies attached to the brush border (X 10,000; ileum).
The absorption of nutrients from the diet is the result of a series of complicated processes in the gastrointestinal tract. In its simplest form it can be considered as processes of hydrolysis and fermentation with the end product of each being absorbed by the enterocytes. Before being absorbed, ingested proteins and carbohydrates are degraded in the intestine by pancreatic enzymes.
Further digestion occurs by a variety of enzymes abundant at the brush border membrane, generating the small molecules (monosaccharides, amino acids, di- and tripeptides) that can be absorbed by the enterocytes. Transport of these molecules through the intestine occurs mainly by the various membrane carrier proteins, for example the sodium-glucose transporter which carries glucose and the PepT1 transporter that transfers di- and tripeptides.
Nutrients, vitamins and minerals, moving across the enterocyte from the intestinal lumen to the blood, must traverse an unstirred layer of mucus, a glycocalyx (network of glycoproteins overlaying the apical surface), the microvilli membrane (the apical membrane of the enterocyte), the basolateral cell membrane, and finally the wall of the blood capillary or the lymphatic vessel.
Digestion and absorption at the brush border
‘Mature’ enterocytes, usually located at the upper section of the villus, are fully equipped for their digestive and absorptive function. It has been established that mature and active enterocytes bear microvilli and have an outer coat called a glycocalyx. The core proteins of the glycocalyx are anchored in the plasma membrane of the enterocyte where part of these glycoproteins represent digestive enzymes and absorptive transmembrane carrier proteins. The mature enterocyte also expresses disaccharidase and aminopeptidase activities along with expression and activity of major transporters including the sodium-glucose transporter and Na+K+ATPase (Buddington, 1997; Wieser, 1973; Traber et al., 1991).
Major changes in the expression and localization of the functional brush border proteins were found in the chick at early age and calculation of the total activity of brush border enzymes on a regional basis yields activity curves for disaccharides, γ-glutamyl transferase and alkaline phosphatase that increase with age in a curvilinear pattern (Uni et al., 1998a; 1999).
From the end of the first week post-hatch, the activity of the brush border enzymes per mass of intestine is closely correlated with the number of enterocytes per villus, suggesting that the amount of enzyme activity expressed per enterocyte does not change greatly with age. However, it has been shown that maturation and activity at the brush border region is subject to dietary manipulation and to changes in feeding regimens.
Following are three examples:
In poultry and in rats, a vitamin A-deficient diet led to reduced activities of several brush border enzymes and transporters as vitamin A influences proliferation and maturation of enterocyte cells by causing hyperplasia (Uni et al., 1998b; Reifen et al., 1998 ).
Tako demonstrated that administration of exogenous nutrients and minerals into the amniotic fluid of the pre-hatch embryo led to elevated mRNA levels of the genes encoding brush border enzymes and transporters, followed by their increased biochemical activities (Tako et al., 2004; 2005).
Experiments examining the effect of mannan oligosaccharide (Bio-Mos®, Alltech Inc.) supplementation of the broiler diet on the brush border functionality revealed elevated gene expression and activity of several major brush border enzymes and transporters, compared to control (no supplementation) and antibiotic growth promoter (AGP)- supplemented diets (Figure 2).
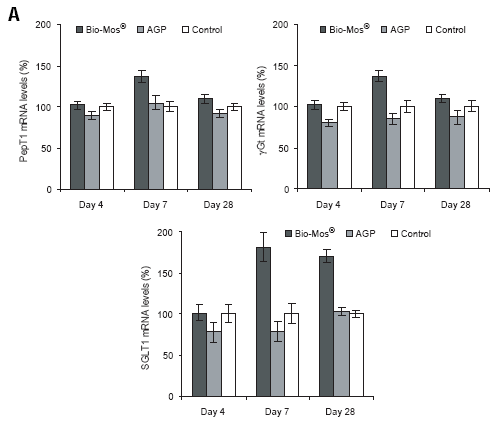
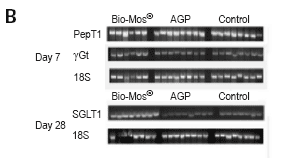
Figure 2.The effect of Bio-Mos® and an AGP (virginiamycin) on mRNA expression of the brushborder transporters peptide transporter (PepT1), γ-Glutamyl transferase (γGT) and sodium/glucose cotransporter (SGLT-1) in the chicken small intestine. (A) Changes in PepT1, γGT and SGLT1 mRNA expression were measured by semi-quantitative RT-PCR, and expression of 18S rRNA was used as a housekeeping gene. Values are means ± SEM, n=8. Means without a common letter differ, P<0.05. (B) Representative picture of PepT1, γGT and SGLT1 mRNA and 18S rRNA amplification at day 7 and day 28 in the jejunum of the Bio-Mos®, AGP and control groups.
Mucin production and secretion at the brush border
The mucus layer, a viscous secretion synthesized by goblet cells, is a continuous layer about 400 μm in thickness that covers the brush border region. The viscosity results from non-covalent interactions between large and highly hydrated glycoconjugates. The mucins have a small peptide core with many long, often branched, side chains of sugars. At the end of their synthetic pathway sulphate or sialic acid may be added to mucin terminal sugars, resulting in acidic mucins that are more resistant to microbial degradation (Mantle and Allen, 1989). It has been proposed that acidic mucins protect against bacterial translocation because they appear less degradable by bacterial and host proteolytic enzymes.
The mucus layer is a component of the innate host immune response and acts as the first defense barrier between the intestinal surface and the intestinal luminal content. The ability of mucus to adhere to the intestinal surface makes it a biological lubricant, protecting the intestinal wall from mechanical damage by dietary constituents. It also protects the epithelium from the corrosive action of the acidic gastric juices and from proteolysis by pancreatic digestive enzymes. Additionally, the mucus layer acts as a medium for nutrient transport between the luminal contents and the epithelial cells.
Mucin glycoproteins undergo polymerization and as a result form a network of mucin molecules that traps other components of the mucus layer such as water, various molecules and cellular macromolecules, electrolytes, microorganisms, and sloughed cells. A number of protective mucus layer-associated proteins are either co-secreted with mucins or interact with the mucous environment to perform their protective functions.
Nutritional factors (high dietary fiber or protein content) and regimens such as fasting or malnutrition affect both mucin biosynthesis and secretion in the small intestine and lead to changes in the luminal content of mucin and in the thickness of the mucus layer (Sharma et al., 1997; Montagne et al., 2004; Piel et al., 2005; Morita et al., 2006).
These changes in turn influence intestinal defensive properties (Deplancke and Gaskins, 2001; Montagne et al., 2004) but do not disturb nutrient absorption (Morita et al., 2006) and do not affect the apparent digestibility of the diet (Montagne et al., 2004).
A study examining the effect of Bio-Mos® and an AGP (virginiamycin) on the mucin dynamics in the chicken intestine showed that the Bio-Mos® supplement did not change small intestinal morphology. However, Bio-Mos® caused increased production of mucin as indicated by increased MUC 2 mRNA expression, increased thickness of the mucin adherent layer and increased dimensions of goblet cells in all small intestinal segments compared to the other groups.
Enterocyte-mucin-bacteria interactions at the brush border
The dynamic nature of the brush border region is due to the cross talk and interactions between the enterocyte apical membrane, the mucin molecules and the intestinal microflora. The dynamic status is reflected in the ongoing renewal of epithelial cells, the processes of polarization and maturation of enterocyte cells (by acquiring the abilities for digestion and absorption via various enzymes and transporters) and the production of mucus from goblet cells.
Once mucin is synthesized in the goblet cells and secreted to the intestinal surface, it forms an adherent layer that undergoes continuous degradation and renewal (Forstner et al., 1995). The total amount of mucin glycoprotein present in the small intestine is influenced both by the rate of mucin synthesis and secretion and by the contribution of the microflora to mucin degradation. Since mucins are resistant to gastrointestinal proteolytic enzymes, the role of the microflora in mucus degradation is of major importance.
There are many bacterial species that possess mucin-degrading glycosidases and glycosulphatases (Robertson and Wright, 1997; Salyers et al., 1977; Slomiany et al., 1987; Variyam and Hoskins, 1981). An ability to enzymatically degrade mucus has been documented in both pathogens and commensals and appears to be a common trait among bacteria (Corfield et al., 2001). Enzymatic digestion of the mucus coat provides access to readily available sources of carbon and energy and enables bacteria to reach the epithelial surface. The intestinal microflora interact with the mucus layer and influence processes of mucin synthesis and secretion ((Deplancke and Gaskins, 2001). Both Grampositive and Gram-negative bacteria were found to increase mucin gene expression and enhance mucus secretion.
Bacterial colonization is facilitated by ability of microbes to adhere to the target cells.
This ability is associated with the specific bacterial organelle, the pilli, which extends from the surface of Gram-negative organisms. Pilli components include lectins that possess mannose-binding activity (Isberg and Barnes, 2002).
The presence of mannan oligosaccharides in the gastrointestinal tract may lead to the competitive exclusion of the selective bacterial species and therefore change the microenvironment surrounding the brush border membrane. These changes may lead to increased concentrations of the short chain fatty acids (SCFA) close to the intestinal surface. SCFA are important determinants of intestinal mucosal growth. Two main pathways regulating gene expression are today attributed to butyrate: butyrate regulates expression of numerous genes through the histone deacetylase (HDAC) pathway (Della Ragione et al., 2001), but butyrate metabolism could also be needed for the modulation of the expression of other genes (Leschelle et al., 2000).
In studies on rats Tapenden et al. (1997) showed that physiological concentrations of butyrate up-regulated the expression of key enterocyteassociated nutrient transporters either directly or via increased release of glucagon-like peptide 2 (Tappenden et al., 2003; 1997). This leads to the conclusion that enterocyte functionality via up-regulation of genes, coding for brush border enzymes and transporters, may occur as a direct response to microbial colonization.
References
Buddington, K.R. 1997. Intestinal nutrient transport during the life history of swine. In: Digestive Physiology in Pigs (J.-P. Laplace, C. Fevrier and A. Barbeau, eds). EAAP publication No. 88, St Malo, France, pp. 103-112.
Corfield, A.P., D. Carroll, N. Myerscough and C.S. Probert. 2001. Mucins in the gastrointestinal tract in health and disease. Frontiers Biosci. 6:D1321-1357.
Forstner, J.F., M.G. Oliver and F.A. Sylvester. 1995. Production, structure and biologic relevance of gastrointestinal mucins. In: Infections of the Gastrointestinal Tract (R.L. Guerrant, ed). Raven Press, New York, NY, USA, pp. 71-88.
Granger, B. and R. Baker. 1950. Electron microscopic investigation of the striated border of intestinal epithelium. Anat. Rec. 107:423-441.
Della Ragione, F., V. Criniti, V. Della Pietra, A. Borriello, A. Oliva, S. Indaco, T. Yamamoto and V. Zappia. 2001. Genes modulated by histone acetylation as new effectors of butyrate activity. FEBS Lett. 499(3):199-204.
Deplancke, B. and H.R. Gaskins. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73(6):1131S-1141S.
Isberg, R.R. and P. Barnes. 2002. Dancing with the host: flow-dependent bacterial adhesion. Cell 110(1):1-4.
Leschelle, X., S. Delpal, M. Goubern, H.M. Blottiere and F. Blachier. 2000. Butyrate metabolism upstream and downstream acetyl-CoA synthesis and growth control of human colon carcinoma cells. Eur. J. Biochem. 267(21):6435-6442.
Mantle, M. and A. Allen. 1989. Gastrointestinal mucus. In: Gastrointestinal Secretion (J.S. Davison, ed). University Press, London, pp. 202-229.
Montagne, L., C. Piel and J.P. Lalles. 2004. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr. Rev. 62(3):105-114.
Morita, T., H. Tanabe, H. Ito, S. Yuto, T. Matsubara, T. Matsuda, K. Sugiyama and S. Kiriyama. 2006. Increased luminal mucin does not disturb glucose or ovalbumin absorption in rats fed insoluble dietary fiber. J. Nutr. 136(10):2486-2491.
Piel, C., L. Montagne, B. Seve and J.P. Lalles. 2005. Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation. J. Nutr. 135(1):86-91.
Reifen, R., G. Zieger and Z. Uni. 1998. Effect of vitamin A on small intestinal brush border enzymes in rat. Intl. J. Vitaminol. Res. 68:281-286.
Robertson, A.M. and D.P. Wright. 1997. Bacterial glycosulphatases and sulphomucin degradation. Canadian J. Gastroenterology 11:361-366.
Salyers, A.A., S.E. West, J.R. Vercellotti and T.D. Wilkins. 1977. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 34:529-533.
Sharma, R., F. Fernandez, M. Hinton and U. Schumacher. 1997. The influence of diet on the mucin carbohydrates in the chick intestinal tract. Cell Mol. Life Sci. 3(11-
12):935-942.
Slomiany, B.L., J. Bilski, J. Sarosiek, V.L. Murty, B. Dworkin, K. VanHorn, J. Zielenski and A. Slomiany. 1987. Campylobacter pyloridis degrades mucin and undermines gastric mucosal integrity. Biochem. Biophysical. Res. Commun. 144:307-314.
Tako, E., P. Ferket and Z. Uni. 2004. Effects of in ovo feeding of carbohydrates and ßhydroxy-ß-methylbutyrate on the development of chicken intestine. Poult. Sci. 83:2023-2028.
Tako, E., P. Ferket and Z. Uni. 2005. Changes in chicken intestinal zinc exporter (ZnT1) mRNA expression and small intestine functionality following an intra amniotic zincmethionine (ZnMet) administration. J. Nutr. Biochem. 16:339-346.
Tappenden, K.A., A.B. Thomson, G.E. Wild and M.I. McBurney. 1997. Short-chain fatty acid-supplemented total parenteral nutrition enhances functional adaptation to intestinal resection in rats. Gastroenterology 112(3):792-802.
Tappenden, K.A., D.M. Albin, A.L. Bartholome and H.F. Mangian. 2003. Glucagonlike peptide-2 and short-chain fatty acids: a new twist to an old story. J. Nutr. 133(11):3717-3720.
Traber, P.G., D.L. Gumucio and W. Wang. 1991. Isolation of intestinal epithelial cells for the study of differential gene expression along the crypt-villus axis. Am. J. Physiol. 260:G895-903.
Uni, Z., S. Ganot and D. Sklan. 1998a. Posthatch development of mucosal function in broiler small intestine. Poult. Sci. 77:75-82.
Uni, Z., G. Zieger and R. Reifen. 1998b. Vitamin A deficiency induced morphometric changes and decreased functionality in chicken small intestine. Brit. J. Nutr. 80:401- 407.
Uni, Z., Y. Noy and D. Sklan. 1999. Posthatch development of small intestinal function in the poult. Poult. Sci. 78:215-222.
Variyam, E.P. and L.C. Hoskins. 1981. Mucin degradation in human colon ecosystems. Degradation of hog gastric mucin by fecal extracts and fecal cultures. Gastroenterology 81:751-758.
Weiser, M.M. 1973. Intestinal cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J. Biol. Chem. 248:2536-2541.
Author: ZEHAVA UNI
Department of Animal Science, Faculty of Agriculture, Hebrew University, Israel
Related topics
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.







.jpg&w=3840&q=75)