Intestinal Integrity in Broilers
Feed conversion and weight gain relative to the anticipated energy formulation of the diet are sensitive indicators of a problem that involve intestinal health. Total water consumption should be measured and recorded daily for each farm, or preferably, each house. Clinical assessment of the flock includes assessment of fecal droppings. Poorly formed feces without a urate cap, and feces with excessive mucus, hemorrhage, red or yellow discoloration, and intact feed particles are indicators of poor intestinal health. Fecal staining of feathers around the vent is a sign of diarrhea. Diarrhea leads to excessive litter moisture which can result in ammonia intoxication.
At necropsy, lesions can be evaluated for severity and a semiquanitative scoring system applied. Lesion severity should be defined in a written protocol for continuity of data collection. One method used by the author is 1= normal, 2= minimal-mild, 3=moderate, and 4=marked-severe. Using a scoring a system beginning with 1=normal allows for nonparametric statistical analysis. Many parameters can be valuable if measured and recorded consistently over time. Oral lesions, crop lesions, gizzard erosion, proventriculitis, coccidiosis at all levels of the intestine, intestinal mucus, undigested feed in the distal digestive tract, focal or diffuse necrotizing lesions, weight and length of the intestine, and helminthe parasites are some examples of parameters and lesions that can be scored, recorded, and analyzed. For research trials, the viscosity of intestinal contents, and the breaking (shearing) strength of the intestine are commonly measured. For microscopic examination, the intestine should be evaluated in a consistent manner. The intestine should be uniformly collected and fixed in neutral-buffered formalin, with caution to not exceed the fixation capacity of the formalin. The author's preference is that the intestine is collected in 1-cm lengths, at the same location and kept intact (not opened). The intestine can be gently agitated when placed in the formalin to dislodge the content and allow penetration of the formalin to properly fix the tips of the villi. Collect the tissue within 5 minutes of euthanasia to prevent autolysis of the tips of the villi.
The author recommends 1-cm lengths of intestine be collected from 1) the descending duodenum (with pancreas attached), 2) middle - slightly proximal to the yolk stalk diverticulum, and 3) cecum - a section through the distal small intestine and the middle of the cecal pouches. Proventriculus and liver are also recommended for examination. Routine histologic assessment includes grading for coccidiosis, crypt hyperplasia, villous atrophy, and inflammatory cells in the lamina propria. Other lesions can be included such nematode parasites, adherent bacteria, long segmented filamentous organisms, and cystic crypts. For research trials, intraepithelial leukocytes, goblet cells, and mucus production can also be measured in the intestine. For proventriculus, inflammation of the mucosa and the glands are scored. For liver, inflammation is scored for parenchyma and bile ductules.
No single measurement provides a complete assessment of intestinal integrity. Multiple measurements collected over time with relatively uncomplicated analysis, can provide valuable information about the cause and pathogenesis of intestinal disease.
Current Observations in Broiler Intestinal Health
The runting stunting syndrome (RSS) remains a problem, based on the presence of histogic lesions and the recovery of enteric viruses, including astrovirus, rotavirus, reovirus, and parvovirus. In the author's experiences in Alabama, the current pattern is one of low-level or subclinical infection, that emerges as sporadic clinical disease when the correct combination of events occurs; however, the specific events are poorly defined. The typical disease five years ago involved broilers 7-12 day of age. Today, it is possible to see lesions and recover virus at 15-18 days, and in older birds, nonspecific enteritis as the sequela at 20-25 days of age. Cases with nonspecific enteritis and detectable RSS-associated viruses have been observed in broilers at 42 days of age, consistent with prolonged infectivity by the viruses. An estimated 30 to 50% of the cases of RSS have one or more birds with mild to moderate lymphocyte depletion from either bursa or thymus. Cases have been observed in which lesions of RSS, or the sequela, and atrophied lymphoid organs are present in flocks undergoing infectious bursal disease challenge and acute necrotizing bursitis. These flocks are experiencing serial expression of potentially immunosuppressive diseases. Cases of RSS associated with necrotic enteritis (Clostridium) have been seen in broilers as young as 14 days.
Eimeria maxima is the predominant species of coccidia in broilers in cases presented to the Alabama diagnostic laboratories. Histologic assessment of intestines reveals that E. maxima coccidiosis often occurs in conjunction with infectious bursal challenge and onset of acute necrotizing bursitis. Flocks in which these two diseases occur simultaneously have vague clinical signs of increased mortality, huddling behavior suggestive of fever or chilling, and poorly formed feces. In the author's experience, most cases of necrotic enteritis are associated with moderate to severe Eimeria maxima coccidiosis.
The most common lesions in the proventriculus include nonspecific inflammation of the mucosa, and lymphocytic adenitis of the glands, indicative of transmissible viral proventriculitis. Although the general prevalence of transmissible proventriculitis has declined in the last two years, it remains a subclinical problem that can be observed in clinically normal, and in sick birds. Nonspecific enteritis can accompany transmissible viral proventriculitis. Inflammation of the proventriculus mucosa is relatively nonspecific. It is increased in transmissible proventriculitis, but in many cases the cause is not known. Irritating substances in the feed, possibly mycotoxins or other biotoxins, or litter consumption may be involved but are largely not defined by routine diagnostics. Fissures and roughening of the gizzard lining (gizzard erosion) occurs with litter consumption, and in conjunction with lesions in the proventriculus. Microscopic examination of affected gizzards reveals that the mucosa is inflamed, typically with lymphocytes and plasma cells, and also heterophils. Hemorrhage can often be seen at the most distal region of the mucosal folds. The overlying koilin is poorly fused with layers of exfoliated epithelial cells, free erythrocytes, and spent inflammatory cells in the defects and associated with laminar and vertical defects in formation. It is possible to induce lesion of this type by experimental inoculation of broilers with adenovirus, with the development intranuclear inclusion bodies in the mucosa. This is a relatively rare lesion in broilers today. Adenovirus has the potential to induce gizzard lesions, but evidence of a significant role is lacking, based on diagnostic investigation.
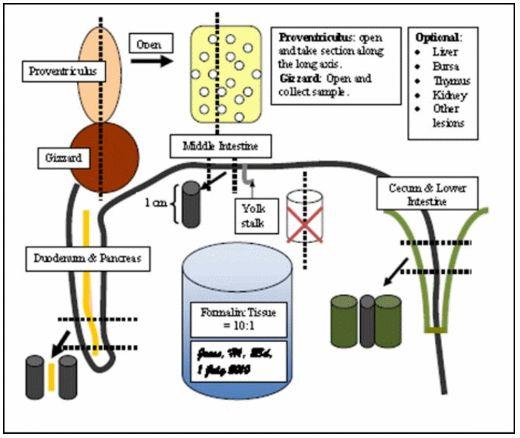
Figure 1. A guide to collection of digestive tissues for histopathology. Select tissues from 3 to 5 birds per flock that are representative of the problem. If mortality is a problem, select tissue from recent mortality and sick live birds. Use neutral-buffered formalin: formalin:tissue = 10:1 (avoid diluted hatchery formaldehyde). Collect proventriculus (gizzard optional), duodenum with pancreas, middle small intestine (in front of the yolk stalk), lower small intestine and cecum (at middle of cecum). Liver, bursa, thymus, kidney and other digestive tract tissues can be collected as indicated. Intestinal segments should be 1-cm in length; do not open - leave intact tubular shape. Close the formalin container and gently agitate to loosen the intestinal content and allow formalin perfusion and fixation. Label the formalin container (not the lid): Farm, flock, age, date of collection.
Dear Colleague
Thank you very much for your article. These are mainly parasitic diseases (coccidiosis, histomonosis) that cause pathological changes in the gut of poultry. Therefore, the production performance (live body weigt, feed Intake,FCR, dressing percentage with out giblets) are then very low.
Best regards
One can expect to get maximum feed converstion from a good quality feed when the digestive system of the flock is fully developed and in good health. Intestinal tract is the main site for digestion and absorption of feed. The author provide a very good guide to monitor intestinal integrity and gut health of the flock. Very useful aticle for people involved in poultry production.
Dear Sir,
We have gone through the Coccidia Scoring methods by the same way but your article represents to overall digestive tract integrity.
It´s a very useful tool to evaluate the Gut health at farm level. If you have such an analytical data in your hand with different parameters then to achieve the FCR of 1.5 for 2.0 kg body weight is at your sight.
I hope this will help our indian farmers/integrators/technicians to achive the above said goal by applying your thoughts & ideas.
I am really thankful to you for providing such useful information & also expecting to share your ideas & thoughts vide Engormix for the wellbeings of world poultry industry.
Thanks & regards,
Dr Jaydip Mulik

















.jpg&w=3840&q=75)