Influence of Graded Levels of L-Theanine Dietary Supplementation on Growth Performance, Carcass Traits, Meat Quality, Organs Histomorphometry, Blood Chemistry and Immune Response of Broiler Chickens
Author details:
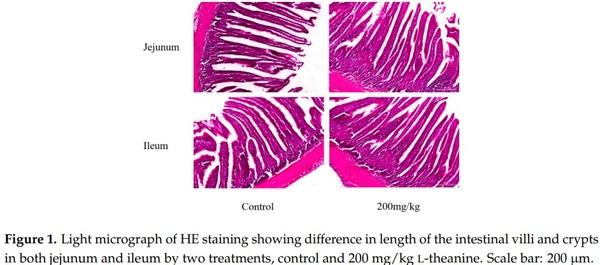
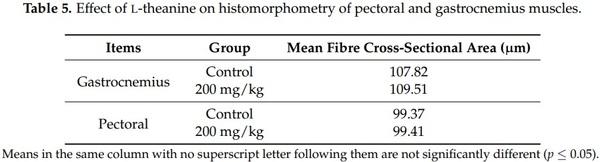
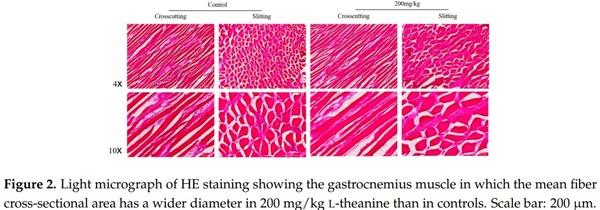
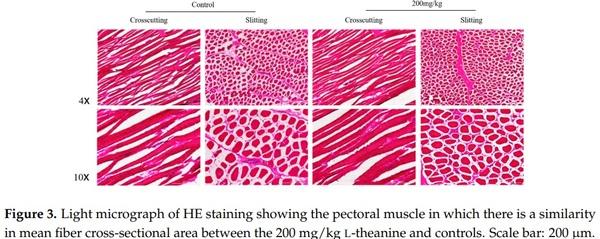
1. Bhatti, M. Emerging prospects of poultry production in Pakistan at the dawn of 21st century. Vet. News Views
2011, 6, 24–30.
2. Dhama, K.; Latheef, S.K.; Mani, S.; Samad, H.A.; Karthik, K.; Tiwari, R.; Khan, R.U.; Alagawany, M.;
Farag, M.R.; Alam, G.M. Multiple beneficial applications and modes of action of herbs in poultry health and production—A review. Int. J. Pharmacol. 2015, 11, 152–176. [CrossRef]
3. Saeed, M.; Baloch, A.R.; Wang, M.; Soomro, R.N.; Baloch, A.M.; Bux, B.A.; Arian, M.A.; Faraz, S.S.;
Zakriya, H.M. Use of Cichorium intybus Leaf extracts as a growth promoter, hepatoprotectant and immune modulent in broilers. J. Anim. Prod. Adv. 2015, 5, 585–591. [CrossRef]
4. Ayssiwede, S.; Dieng, A.; Bello, H.; Chrysostome, C.; Hane, M.; Mankor, A.; Dahouda, M.; Houinato, M.;
Hornick, J.; Missohou, A. Effects of Moringa oleifera (Lam.) leaves meal incorporation in diets on growth performances, carcass characteristics and economics results of growing indigenous senegal chicken.
Pak. J. Nutr. 2011, 10, 1132–1145. [CrossRef]
5. El-Hack, M.E.A.; Alagawany, M.; Farag, M.R.; Tiwari, R.; Karthik, K.; Dhama, K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharmacol. 2016,
12, 232–248. [CrossRef]
6. Al-Basher, G.; Ajarem, J.S.; Allam, A.A.; Mahmoud, A.M. Green tea protects against perinatal nicotine-induced histological, biochemical and hematological alterations in mice off spring. Int. J. Pharmacol.
2017, 13, 109–121.
7. Lin, X.; Lin, C.-H.; Zhao, T.; Zuo, D.; Ye, Z.; Liu, L.; Lin, M.T. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem. Biol. Interact. 2017,
265, 47–54. [CrossRef] [PubMed]
8. Akinloye, O.A.; Somade, O.T.; Akindele, A.S.; Adelabu, K.B.; Elijah, F.T.; Adewumi, O.J. Anticlastogenic and hepatoprotective properties of ginger (Zingiber officinale) extract against nitrobenzene-induced toxicity in rats. Roman. J. Biochem. 2014, 51, 3–15.
9. Alagawany, M.; El-Hack, M.E.A.; El-Kholy, M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. 2016, 23, 6774–6782. [CrossRef] [PubMed]
10. Saeed, M.; Abd El-Hack, M.; Alagawany, M.; Arain, M.; Arif, M.; Mirza, M.; Naveed, M.; Chao, S.; Sarwar, M.;
Sayab, M. Nutritional and Healthical Aspects of Yacon (Smallanthus sonchifolius) for Human, Animals and
Poultry. Int. J. Pharmacol. 2017, 13, 361–369. [CrossRef]
11. Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review.
Chin. Med. 2010, 5, 13. [CrossRef] [PubMed]
12. Abdo, Z.M.; Hassan, R.; El-Salam, A.A.; Helmy, S.A. Effect of adding green tea and its aqueous extract as natural antioxidants to laying hen diet on productive, reproductive performance and egg quality during storage and its content of cholesterol. Egypt. Poult. Sci. J. 2010, 30, 1121–1149.
13. Kojima, S.; Yoshida, Y. Effects of green tea powder feed supplement on performance of hens in the late stage of laying. Int. J. Poult. Sci. 2008, 7, 491–496. [CrossRef]
14. Eschenauer, G.; Sweet, B.V. Pharmacology and therapeutic uses of theanine. Am. J. Health Syst. Pharm. 2006,
63, 28–30. [CrossRef] [PubMed]
15. Deng, W.W.; Ogita, S.; Ashihara, H. Distribution and biosynthesis of theanine in Theaceae plants.
Plant Physiol. Biochem. 2010, 48, 70–72. [CrossRef] [PubMed]
16. Tian, X.; Sun, L.; Gou, L.; Ling, X.; Feng, Y.; Wang, L.; Yin, X.; Liu, Y. Protective effect of L-theanine on chronic restraint stress-induced cognitive impairments in mice. Brain Res. 2013, 1503, 24–32. [CrossRef] [PubMed]
17. Kurihara, S.; Shibahara, S.; Arisaka, H.; Akiyama, Y. Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of L-cystine and L-theanine. Vet. Med. Sci. 2007, 69, 1263–1270.
[CrossRef]
18. Yang, H.; Li, W.; Yu, H.; Yuan, R.; Yang, Y.; Pung, K.; Li, P.; Xue, L. Physiological effects of L-Theanine on
Drosophila melanogaster. Molecules 2013, 18, 13175–13187. [CrossRef] [PubMed]
19. Li, C.; Tong, H.; Yan, Q.; Tang, S.; Han, X.; Xiao, W.; Tan, Z. L-Theanine Improves Immunity by Altering
TH2/TH1 Cytokine Balance, Brain Neurotransmitters, and Expression of Phospholipase C in Rat Hearts.
Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 662–669. [CrossRef]
20. Yin, C.; Gou, L.; Liu, Y.; Yin, X.; Zhang, L.; Jia, G.; Zhuang, X. Antidepressant-like effects of L-theanine in the forced swim and tail suspension tests in mice. Phytother. Res. 2011, 25, 1636–1639. [CrossRef] [PubMed]
21. Takeda, A.; Tamano, H.; Suzuki, M.; Sakamoto, K.; Oku, N.; Yokogoshi, H. Unique induction of CA1
LTP components after intake of theanine, an amino acid in tea leaves and its effect on stress response.
Cell. Mol. Neurobiol. 2012, 32, 41–48. [CrossRef] [PubMed]
22. Wen, H.; Wei, S.; Zhang, S.; Hou, D.; Xiao, W.; He, X. Effects of L-theanine on performance and immune function of yellow-feathered broilers. Chin. J. Anim. Nutr. 2012, 24, 1946–1954.
23. Hwang, Y.; Park, B.; Lim, J.; Kim, M.; Song, I.; Park, S.; Jung, H.; Hong, J.; Yun, H. Effects of β-Glucan from
Paenibacillus polymyxa and L-theanine on Growth Performance and Immunomodulation in Weanling Piglets.
Asian-Australas. J. Anim. Sci. 2008, 21, 1753–1759. [CrossRef]
24. Williams, J.; Kellett, J.; Roach, P.D.; Mckune, A.; Mellor, D.; Thomas, J.; Naumovski, N. L-theanine as a functional food additive: Its role in disease prevention and health promotion. Beverages 2016, 2, 13.
[CrossRef]
25. Biswas, A.H.; Wakita, M. Effect of dietary Japanese green tea powder supplementation on feed utilization and carcass profiles in broilers. J. Poult. Sci. 2001, 38, 50–57. [CrossRef]
26. Sarker, M.; Kim, G.; Yang, C. Effect of green tea and biotite on performance, meat quality and organ development in Ross broiler. Egypt. Poult. Sci. J. 2010, 30, 77–88.
27. Erener, G.; Ocak, N.; Altop, A.; Cankaya, S.; Aksoy, H.M.; Ozturk, E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian-Australas. J. Anim. Sci.
2011, 24, 1128–1135. [CrossRef]
28. Uuganbayar, D. A Study on the Utilization of Green Tea for Laying Hens and Broiler Chicks. Ph.D. Thesis,
Sunchon National University, Suncheon, South Korea, 2004.
29. Yang, C.; Yang, I.; Oh, D.; Bae, I.; Cho, S.; Kong, I.; Uuganbayar, D.; Nou, I.; Choi, K. Effect of green tea by-product on performance and body composition in broiler chicks. Asian-Australas. J. Anim. Sci. 2003, 16,
867–872. [CrossRef]
30. Kamath, A.B.; Wang, L.; Das, H.; Li, L.; Reinhold, V.N.; Bukowski, J.F. Antigens in tea-beverage prime human
Vγ2Vδ2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc. Natl.
Acad. Sci. USA 2003, 100, 6009–6014. [CrossRef] [PubMed]
31. Afsharmanesh, M.; Sadaghi, B. Effects of dietary alternatives (probiotic, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. Comp. Clin. Pathol. 2014, 23, 717–724. [CrossRef]
32. Bukowski, J.F.; Morita, C.T.; Brenner, M.B. Human cd T cells recognise alkylamines derived from microbes, edible plants, tea: Implications for innate immunity. Immunity 1999, 11, 57–65. [CrossRef]
33. Zheng, G.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004, 18, 55–62. [PubMed]
34. Bukowski, J.F.; Percival, S.S. L-theanine intervention enhances human γδ T lymphocyte function. Nutr. Rev.
2008, 66, 96–102. [CrossRef] [PubMed]
35. Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial.
BMC Complement. Altern. Med. 2011, 11, 15. [CrossRef] [PubMed]
36. Kurihara, S.; Hiraoka, T.; Akutsu, M.; Sukegawa, E.; Bannai, M.; Shibahara, S. Effects of L-cystine and
L-theanine supplementation on the common cold: A randomized, double-blind, and placebo-controlled trial.
J. Amin. Acids 2010, 6, 307475. [CrossRef] [PubMed]
37. Takagi, Y.; Kurihara, S.; Higashi, N.; Morikawa, S.; Kase, T.; Maeda, A.; Arisaka, H.; Shibahara, S.; Akiyama, Y.
Combined administration of L-cystine and L-theanine enhances immune functions and protects against influenza virus infection in aged mice. J. Vet. Med. Sci. 2010, 72, 157–165. [CrossRef] [PubMed]
38. Fletcher, D. Broiler breast meat color variation, pH, and texture. Poult. Sci. 1999, 78, 1323–1327. [CrossRef]
[PubMed]
39. Qiao, M.; Fletcher, D.; Smith, D.; Northcutt, J. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001, 80, 676–680. [CrossRef] [PubMed]
40. Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767.
[CrossRef] [PubMed]
41. Young, J.F.; Stagsted, J.; Jensen, S.K.; Karlsson, A.; Henckel, P. Ascorbic acid, alpha-tocopherol, and oregano supplements reduce stress-induced deterioration of chicken meat quality. Poult. Sci. 2003, 82, 1343–1351.
[CrossRef] [PubMed]
42. Yan, Q.; Tong, H.; Tang, S.; Tan, Z.; Han, X.; Zhou, C. L-theanine administration modulates the absorption of dietary nutrients and expression of transporters and receptors in the intestinal mucosa of rats. BioMed Res. Int.
2017, 2017, 9747256. [CrossRef] [PubMed]
43. Pelícia, K.; Mendes, A.; Saldanha, E.; Pizzolante, C.; Takahashi, S.; Garcia, R.; Moreira, J.; Paz, I.; Quinteiro, R.;
Komiyama, C. Probiotic and prebiotic utilization in diets for free-range broiler chickens. Revista Brasileira de
Ciência Avícola 2004, 6, 99–104. [CrossRef]
44. Mosleh, N.; Shomali, T.; Hamedi, S. Effects of green tea on performance, feed conversion and jejunum (histology) in broilers. Online J. Vet. Res. 2011, 15, 147–154.
45. Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [CrossRef] [PubMed]
46. Abdelqader, A.; Al-Fataftah, A.R. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest. Sci. 2016, 183, 78–83.
[CrossRef]
47. Zhao, D. Review on research status of theanine. Food Sci. 2002, 23, 145–147.
48. Murakami, S.; Kurihara, S.; Titchenal, C.A.; Ohtani, M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: A randomized, double-blind, placebo-controlled trial.
J. Int. Soc. Sports Nutr. 2010, 7, 23. [CrossRef] [PubMed]
49. Ariana, M.; Samie, A.; Edriss, M.A.; Jahanian, R. Effects of powder and extract form of green tea and marigold, and-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in late phase of production. J. Med. Plant Res. 2011, 5, 2710–2716.
50. Abdel-Azeem, F. Green tea flowers (Camellia sinensis) as natural anti-oxidants feed additives in growing
Japanese quail diets. Egypt. Poult. Sci. J. 2005, 25, 569–588.
51. Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [CrossRef]
52. Zheng, G.; Bamba, K.; Okubo, T.; Raj Juneja, L.; Oguni, I.; Sayama, K. Effect of theanine, γ-glutamylethylamide, on bodyweight and fat accumulation in mice. Anim. Sci. J. 2005, 76, 153–157. [CrossRef]
53. El-Deek, A.; Al-Harthi, M. Responses of modern broiler chicks to stocking density, green tea, commercial multi-enzymes and their interactions on productive performance, carcass characteristics, liver composition and plasma constituents. Int. J. Poult. Sci. 2004, 3, 635–645.
54. Xu, X.; Hu, Y.; Xiao, W.; Huang, J.A.; He, X.; Wu, J.; Ryan, E.P.; Weir, T.L. Effects of fermented Camilla sinensis,
Fuzhuan tea, on egg cholesterol and production performance in laying hens. Agric. Food Sci. 2012, 1, 6–10.
55. Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; de Feo, V.; Zia-Ul-Haq, M.
Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 2015, 13, 79. [CrossRef] [PubMed]
56. Yousaf, S.; Butt, M.; Suleria, H.; Iqbal, M. The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct. 2014, 5, 545–556.
[CrossRef] [PubMed]
57. Koo, S.I.; Sang, K.N. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [CrossRef] [PubMed]
58. Tayal, V.; Kalra, B.S. Cytokines and anti-cytokines as therapeutics—An update. Eur. J. Pharmacol. 2008, 579,
1–12. [CrossRef] [PubMed]
59. Li, G.; Ye, Y.; Kang, J.; Yao, X.; Zhang, Y.; Jiang, W.; Gao, M.; Dai, Y.; Xin, Y.; Wang, Q.; et al.
L-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes.
Food Chem. Toxicol. 2012, 50, 363–372. [CrossRef] [PubMed]
60. Jiang, W.; Gao, M.; Sun, S.; Bi, A.; Xin, Y.; Han, X. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem. Biophys. Res. Commun. 2012, 422, 344–350. [CrossRef] [PubMed]
61. Pi, Z.; Wu, Y.; Liu, J. Effect of pretreatment and pelletization on nutritive value of rice straw-based total mixed ration, and growth performance and meat quality of growing Boer goats fed on TMR. Small Rumin. Res.
2005, 56, 81–88. [CrossRef]
62. Liu, Z.; Gu, H.; Gan, L.; Xu, Y.; Feng, F.; Saeed, M.; Sun, C. Reducing Smad3/ATF4 was essential for
Sirt1 inhibiting ER stress-induced apoptosis in mice brown adipose tissue. Oncotarget 2017, 8, 9267–9279.
[CrossRef] [PubMed]
63. Sikandar, A.; Cheema, A.; Younus, M.; Aslam, A.; Zaman, M.; Rehman, T. Histopathological and serological studies on paratuberculosis in cattle and buffaloes. Pak. Vet. J. 2012, 32, 547–551.
64. Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to ImageJ: 25 Years of image analysis. Nat. Methods
2012, 9, 671–675. [CrossRef] [PubMed]
65. Ashraf, S.; Zaneb, H.; Yousaf, M.S.; Ijaz, A.; Sohail, M.U.; Muti, S. Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. J. Anim.
Physiol. Anim. Nutr. 2013, 1, 68–73. [CrossRef] [PubMed]
66. Kiczorowska, B.; Al-Yasiry, A.; Samoli ´nska, W.; Marek, A.; Pyzik, E. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livest. Sci. 2016, 191, 117–124. [CrossRef]
67. Heywood, J.; Mcentee, G.; Stickland, N. In ovo neuromuscular stimulation alters the skeletal muscle phenotype of the chick. J. Muscle Res. Cell Motil. 2005, 26, 49–56. [CrossRef] [PubMed]









.jpg&w=3840&q=75)