INTRODUCTION
Consumption of poultry meat has increased significantly in the United States and currently ranks as the highest consumed among meat species (National Chicken Council, 2020). With increasing chicken meat consumption, greater number of broilers are being raised and processed. Foodborne pathogens Salmonella and Campylobacter are commensals in broiler gastrointestinal tract and, in most cases, do not cause any disease in the birds. Current poultry processing system presents significant risk of cross contamination of the carcasses during primary processing (slaughter) and the meat during any second processing steps (cut up and further processing). FoodNet identified 25,606 infections, 5,893 hospitalizations, and 120 deaths from foodborne sources during 2018 (Tack et al., 2019). FoodNet further reported that the incidence of infections is increasing, including those caused by Campylobacter and Salmonella, which might be partially attributable to the increased use of culture-independent diagnostic tests. The USDA Food Safety Inspection Service (USDA-FSIS) has published revised performance standards for poultry processing for Salmonella and Campylobacter prevalence after chilling step and for chicken parts (USDA FSIS, 2016). The Salmonella and Campylobacter performance standards for broiler carcasses, chicken parts, and comminuted chicken are 9.8 and 15.7%, 15.4, and 7.7%, and 25 and 1.9%, respectively. Further, the USDA-FSIS proposed a change to the Campylobacter detection methodology from direct plating to enrichment, potentially increasing the sensitivity of detection resulting in higher prevalence rates for Campylobacter in poultry.
Poultry processors have incorporated numerous antimicrobial interventions in the process at different locations throughout the first processing (e.g., after picking step) and second processing (e.g., inside outside bird washer, carcass rinses, etc.). While these antimicrobial interventions enhance the efficacy of the process in reducing foodborne pathogen concentrations and prevalence, the main chiller still serves as the primary antimicrobial intervention during broiler processing. Broiler carcasses typically are chilled for 45 min to 1 h 30 min, depending on the process and design of the main chiller. Some processors have incorporated prechillers and postchill dip tanks to enhance the antimicrobial efficacy through use of higher concentrations of antimicrobial (peroxy acetic acid [PAA]), even though for shorter contact times.
While several antimicrobials being evaluated and approved for use in poultry processing, majority of the processors rely on PAA or peracetic acid and use it throughout poultry slaughter and further processing (cut-up). Depending on the location of application, the PAA concentrations of these antimicrobial interventions can vary from 25 to 750 ppm, depending on the location of the intervention step and the application times which can vary from 10 s to 1 h. The PAA is commercially available as an equilibrium mixture of PAA along with hydrogen peroxide and acetic acid; however, the proportion of these mixtures can vary significantly from supplier to supplier, and the regulatory approvals for PAA vary from 50 ppm to 2,000 ppm (USDA-FSIS, 2020). Being in equilibrium mixture with acetic acid, the pH of PAA solutions prepared for use in poultry processing is in the acidic range, with the natural pH of the solutions dependent on the concentration of PAA used for specific application. In some cases, the pH of the PAA solution is further adjusted to lower pH values (up to pH 2.5) using mineral acids such as hydrochloric acid, sulfuric acid, or phosphoric acids and with weak acids such as citric acid to improve the antimicrobial efficacy of the PAA. However, poultry processors prefer using higher pH values as exposure to low pH solutions for extended periods such as in the main chiller can result in moisture loss from the carcass, resulting in lower carcass yields (after chilling). As such, the pH of the PAA solutions is adjusted to values 8.0 or higher using an alkali such as sodium hydroxide. As the pKa of PAA is 8.2 at 25°C (Koubek et al., 1963), the PAA exists in dissociated form at higher pH values. As a weak acid, dissociated PAA (peroxy-acetic anions) cannot penetrate the cell membrane and perturb the internal pH of the cell, the main mechanism of action for inactivation of microorganism by weak organic acids. Therefore, there is a need to evaluate the antimicrobial efficacy of PAA at higher pH values as used in the poultry industry.
The USDA-FSIS mandated that the poultry processors utilize Escherichia coli Biotype I as an indicator for process control and requires sampling of broiler carcasses after chilling step at specific frequency (USDA FSIS, 1996). While all poultry processors conduct E. coli testing on the broiler carcasses, the microorganism has not been used as a surrogate for Salmonella or Campylobacter to evaluate the efficacy of the antimicrobial interventions applied during poultry slaughter and cut-up process.
The USDA-FSIS requires meat and poultry processing operations to validate their Hazard Analysis and Critical Control Points (HACCP) system (USDA FSIS, 2015a). Validation as specified in 9 CFR 417.4(a) (1) requires that meat and poultry processing operations should assemble 2 types of supporting documentation to demonstrate that the requirements are met: 1) scientific and technical support for the HACCP system (design) and 2) inplant validation data (execution; USDA FSIS, 2020). The second element requires collection of inplant validation data, demonstrating the effectiveness of the critical operational parameters. Such inplant validation data can be collected using pathogen prevalence at different locations during processing to demonstrate the efficacy of the antimicrobial interventions and/or critical control points. However, the natural prevalence of such foodborne pathogens of significance may be low and are not uniformly distributed, requiring large sample size. This limitation can be overcome by the use of surrogate microorganisms that demonstrate similar survival characteristics as the foodborne pathogen of significance such as Salmonella and Campylobacter. To be able to use the surrogate microorganisms for this purpose, it is necessary to show the relationship between the 2 microorganisms and that their survival behavior is similar to each other for the specific antimicrobial intervention(s). Marshall et al. (2005) isolated several E. coli Biotype I strains from beef processing operations and showed that 5 strains exhibited characteristics similar to E. coli O157:H7. In subsequent research, Niebuhr et al. (2008) reported that these 5 strains behaved similar to Salmonella when subjected to selected antimicrobial treatments (hot water, hot water–lactic acid, chlorine, and trisodium phosphate), cold storage, and fermentation. These 5 E. coli isolates were deposited in the American Type Culture Collection and can be used for research and for inplant validation of antimicrobial interventions. The USDA FSIS stated that an establishment may use a surrogate indicator organism to measure change, but it should do so only after giving careful consideration to specific precautions outlined in the document (USDA FSIS, 2015b).
While research has been conducted on the behavior of these surrogate microorganisms on beef and antimicrobial interventions applied to beef, they have not been evaluated for their behavior on poultry carcasses/parts and PAA. Thus, there is a need to evaluate their responses to PAA application and whether these surrogate microorganisms can be used as indicators for Salmonella and Campylobacter. The objectives of the study were to (1) evaluate the impact of PAA solution pH on the antimicrobial efficacy against Salmonella, E. coli and Campylobacter and (2) evaluate the ability to use E. coli strains identified as surrogates for Salmonella and Campylobacter on poultry products.
MATERIALS AND METHODS
Bacterial Strains and Inoculum Preparation
A nalidixic acid–resistant strain of Salmonella Typhimurium (STNA) and a gentamicin-resistant strain of Campylobacter coli (CCG) were obtained from the U.S. National Poultry Research Center, United States Department of Agriculture, Athens, GA. Five E. coli biotype I strains (transformed to have rifampin resistance [100 ppm], E. coliRif) were obtained from the Texas A&M Center for Food Safety (Texas A&M University, College Station) and maintained frozen in glycerol (Marshall et al., 2005). Non–rifampin-resistant strains of the same surrogates (BAA-1427, BAA-1428, BAA-1429, BAA-1430, and BAA-1431) were previously deposited in the American Type Culture Collection (Manassas, VA; Niebuhr et al., 2008; Keeling et al., 2009). Similarly, STNA, CCG, and E. coliR glycerol stocks were streaked onto Brilliant Green Sulfa Agar (Difco, Sparks, MD) supplemented with 100 ppm Nalidixic acid (Sigma-Aldrich, St. Louis, MO), Campy Cefex Agar (Neogen Corporation, Lansing, MI) supplemented with 200 ppm gentamycin (CCGA; Sigma-Aldrich) and tryptic soy agar (Difco) supplemented with 100 ppm rifampicin (J6283409 AlfaAesar, Tewksbury, MA). The STNA and E. coliR streaked plates were incubated for 24 h at 35°C ± 1°C, and the CCG streaked plates were placed in a re-sealable plastic bag flushed with a microaerobic environment (5% O2, 10% CO2, and 85% N2) and incubated for 48 h at 42°C ± 1°C. The bacterial cocktails for inoculum were prepared by growing individual colonies of STNA and E. coliR in 2 tryptic soy broth tubes (10 mL) each and incubated for 24 h at 35°C ± 1°C. A loopful of CCG glycerol stocks were streaked on CCGA and incubated at 42°C ± 1°C for 48 h under microaerobic conditions and the process was repeated twice. The active CCG was prepared as a lawn on 8 CCGA plates, incubated as described previously, and the cells from each plate were harvested using 1 mL buffered peptone water/plate and combining the suspension from each plate. The cultures (STNA and E. coliR) were centrifuged at 5,500 x g for 10 min, and the pellet was re-suspended into 5 mL of 0.1% peptone water (PW; Difco) and was repeated twice. The cells were finally resuspended in buffered PW (250 mL) and combined with the CCG suspension to obtain ca. 6 log10 CFU/ mL of STN and E. coliR and 4.5 log10 CFU/mL of CCG.
Preparation of Peroxyacetic Acid Solutions
Appropriate volume of PAA (Zee Company, Chattanooga, TN) was added to prechilled water (4°C) to obtain PAA concentrations of 50, 250, and 500 ppm and adjusted to pH values of 8.2 or 10 for treatments requiring the pH adjustments using sodium hydroxide (NaOH, Mallinckrodt Baker Inc., Phillipsburg, NJ). The PAA concentrations in the solutions were confirmed using Peroxychem Titration kit (LaMotte Company, Ocean City, MD).
Inoculation of Chicken Breast Fillets
Chicken wings before any antimicrobial treatment were obtained from a local commercial processor on each day of the experiment and used the same day. For each treatment, chicken wings (0.45 kg) were placed on a sterile stainless-steel rack and inoculated with the STNR, E. coliR, and CCG cocktail by spraying 5 mL on each side. The stainless-steel rack with the chicken wings was transferred to a Biosafety cabinet for 15 min to allow attachment.
Application of Antimicrobial Treatment
Inoculated chicken wings (0.45 Kg) were placed in a sanitized stainless-steel mesh basket (Model DND095RND120-C04S, Anysizebasket, York, PA) and immersed in appropriate antimicrobial solution in a container. Air agitation of the antimicrobial solution in each container was achieved by pumping air (103.42 kPa) from an air compressor (Model D55146 Air Compressor, Dewalt, Towson, MD) to the bottom by means of 2 concentric circular plastic tubes (ID 0.64 cm,; 11.5 and 15.4 cm, with 1.6 mm holes drilled at 2.5 cm intervals; McMaster-Carr, Elmherst, IL). The concentric plastic tubes were secured to a ceramic plate and placed at the bottom of the container such that the compressed air (bubbling) was uniformly distributed at the bottom of each container. All the antimicrobial solutions were prepared on the day of the experiment. Inoculated wings that were not subjected to any antimicrobial treatment served as a positive control.
Bacterial Enumeration
Treated chicken wings were aseptically transferred into sterile rinse bags (BRB3500; 3M Food Safety, St. Paul, MN) containing 100 mL of chilled buffered peptone water (Difco) supplemented with sodium thiosulfate (0.1%; Acros Organics, Fair Lawn, NJ) to neutralize residual PAA on the chicken wings and rinsed for 1 min. The rinsate was serially diluted in 1) 0.1% PW supplemented with nalidixic acid (100 ppm; PWN), 2) PWR supplemented with rifampicin (100 ppm; PWR), and 3) PW. Appropriate dilutions of PWN and PWR were plated on Petrifilm APC (3M Food Safety, St. Paul, MN) for enumeration of STN and E. coliR and dilutions prepared in PW were plated on CCGA for enumeration of CCG as described by Kumar et al. (2020). The CCGA plates were incubated for 48 h at 45°C under microaerobic conditions, and the Petrifilm APC plates were incubated for 24 h at 35°C.
Experimental Design and Statistical Analyses
A 3 (PAA concentrations; 50, 250 and 500 ppm) x 3 (natural pH, 8.2 and 10) x 2 (exposure times, 10 s or 60 min) experimental design was used. Four independent replications (3 for Campylobacter) of the experiment were performed, as represented by different day of production (chicken wings), PAA solution, and a fresh inoculum. Data were analyzed by analysis of variance using the general linear model procedure of SAS 9.4 (SAS Institute Inc, 2004). Fisher’s least significant difference (a = 0.05) was used to separate means of the microbial populations (log10 CFU/mL).
RESULTS AND DISCUSSION
The pH values of the nonadjusted PAA solutions were 4.46 ± 0.18, 3.67 ± 0.18, and 3.39 ± 0.19 for the 50, 250, and 500 ppm solutions, respectively. Chen et al. (2014) reported an average pH was 3.40 ± 0.2 and 3.32 ± 0.2, respectively, for the PAA solution concentrations of 700 and 1,000 ppm. The PAA concentrate used in the current study was marketed as 25 to 35% hydrogen peroxide, 2.5 to 10% acetic acid, and 2.5 to 10% PAA, with the remaining being water. Preparation of solutions with higher PAA concentrations results in lower pH of the solution as with higher concentrations of PAA, acetic acid concentration will increase concurrently.
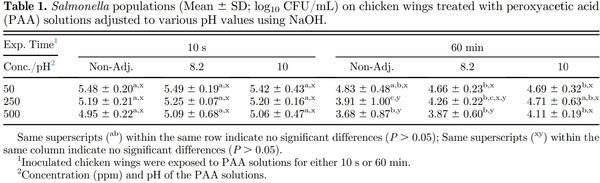
Inoculation of chicken wings with the 3- microorganism cocktail resulted in Salmonella, Campylobacter, and E. coli populations of 6.24, 4.44, and 6.36 log10 CFU/mL, respectively. Immersion of inoculated chicken wings in nonadjusted (pH) PAA solutions of 50, 250, and 500 ppm for 10 s resulted in Salmonella population reductions of 0.76, 1.05, and 1.29 log10 CFU/mL, respectively (Table 1). Immersion of the inoculated product for 60 min resulted in greater reductions in Salmonella populations of 1.41, 3.05, and 2.56 log10 CFU/mL, respectively compared with immersion for 10 s. Similar reductions in Salmonella (P > 0.05) were observed with PAA solutions adjusted to higher pH values, 8.2 and 10. Increasing the PAA concentrations (from 50 ppm to 250 and 500 ppm) did not affect Salmonella reductions (P > 0.05), regardless of the pH of the PAA solution when the chicken wings were immersed for 10 s. The Salmonella reductions were greater (P ≤ 0.05) with higher PAA concentration (500 ppm vs. 50 ppm) and when chicken wings were immersed for longer time (60 min), except for the PAA solution adjusted to pH 10. Kumar et al. (2020) reported reductions in Salmonella populations on breast fillets (boneless, skinless) of 0.57, 0.90, and 1.16 log10 CFU/mL following immersion in PAA solutions of 100, 250, and 500 ppm, respectively for 10 s. These Salmonella reductions were similar to those observed in this study, with 0.76, 1.05, and 1.29 log10 CFU/mL, with chicken wings were immersed in PAA solutions of 50, 250, and 500 ppm, respectively. Bauermeister et al. (2008) reported Salmonella population reductions of ca. 0.9, 1.1, and 1.3 log10 CFU/sample (from chlorine [30 ppm] treated broilers) on broilers when immersed for 1 h in PAA solutions of 25, 100, and 200 ppm, respectively. Scott et al. (2015) reported 1.5 log10 CFU/mL reduction in Salmonella when inoculated chicken wings were immersed in 500 ppm PAA solution for 20 s. Use of chicken wings for evaluating antimicrobial efficacy presents a worst-case scenario as the wing flat is covered entirely with the chicken skin, providing protection to microbial inactivation because of the skin topography with crevices and feather follicles where the microorganism might be protected from exposure to the antimicrobial during immersion or spray application. Also, we evaluated exposure of the product to PAA solutions for 10 s and 60 min to represent typical exposure times experienced by chicken parts and broiler carcasses, respectively.
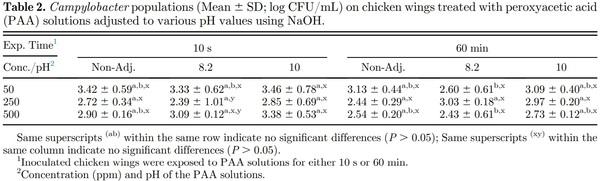
Immersion of inoculated chicken wings in PAA solutions of increasing concentrations resulted in numerically greater Campylobacter reductions; however, they were statistically similar (P > 0.05), within each of the PAA solution pH treatments, regardless of the exposure time (10 s or 60 min; Table 2). Within each of the exposure time (10 s or 60 min), the Campylobacter populations were similar for all the PAA solution pH values within each PAA solution concentration (50, 250, and 500 pm). Kumar et al. (2020) reported Campylobacter reductions of 0.71 and 1.25 log10 CFU/mL on chicken breast fillets (skinless, boneless) when immersed in PAA solutions of 240 and 500 ppm, respectively. Chen et al. (2014) reported similar reductions in Campylobacter populations (ca. 1.0 log10 CFU/g) on boneless chicken breast and skin-on thighs on exposure to PAA solutions of 700 ppm.
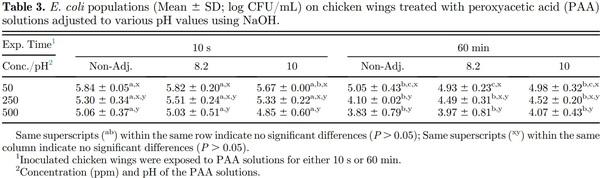
Greater E. coli reductions were observed when the inoculated chicken wings were immersed in PAA solutions for 10 s with increasing PAA concentrations within the same pH of the PAA solution (Table 3). While numerical reductions were greater with increasing PAA concentrations, significant differences (P ≤ 0.05) in E. coli populations were only observed between 50 and 500 ppm for each of the PAA solution pH. Immersion of inoculated chicken wings for 60 min resulted in greater (P ≤ 0.05) E. coli reductions compared with respective PAA solution pH-concentration treatment combinations (except for pH 10 PAA solution). Literature on the efficacy of PAA on reduction of E. coli populations on poultry products is lacking. While poultry processors are required to sample the broiler carcasses for E. coli Biotype I populations after chilling, sampling is not conducted after antimicrobial interventions to allow evaluation of the efficacy of those intervention steps. The 5 E. coli Biotype I strains used in the current study were evaluated for their behavior in response to antimicrobial interventions applied in the beef industry such as hot water sprays, organic acid sprays (lactic acid), combination of hot water and lactic acid sprays, chlorinated water, and trisodium phosphate solutions (Niebuhr et al., 2008). The 5 E. coli Biotype I strains behaved similar to Salmonella and E. coli O157:H7 and are approved by USDA FSIS for use in conducting inplant validation studies to demonstrate efficacy of antimicrobial interventions (USDA FSIS, 2015b). While the practice of inplant validation studies is not common in the poultry industry, the use of nonpathogenic microorganism as a surrogate for the foodborne pathogen Salmonella in meat processing operations should be practiced, similar to the beef and swine processing operations. Inoculation of relatively high populations of the surrogate microorganisms immediately before processing will result in similar stresses imposed on the microorganisms during processing (such as temperature during scalding, antimicrobials, and other chemicals at inside outside bird washer steps, etc.). Further, use of high and similar concentrations of the surrogate microorganisms will allow comparisons in reductions of surrogate populations at each processing step or operation and throughout the slaughter/cut-up/further processing without the limitation of using qualitative methods (such as methods used to determine prevalence of a microorganism, e.g., Salmonella) for foodborne pathogens which are present in the product at low populations with high flock to flock variability in populations and/or prevalence. While it is possible to use naturally occurring Salmonella on broiler carcasses or the chicken parts, the concentration of Salmonella can be very low, making it difficult to evaluate the reduction in populations achieved at each of the antimicrobial intervention steps. Proper use of a surrogate microorganism allows for a better measurement to determine the efficacy of the antimicrobial intervention steps rather than relying on pathogen prevalence data. A plot of the Salmonella and E. coli populations on the chicken wings immersed in PAA solutions of various concentrations, exposure times, and pH values indicate a good correlation (0.93), suggesting that the E. coli surrogate strains can be used in lieu of Salmonella for conducting inplant validation studies (Figure 1). Poultry processors are incorporating antimicrobial interventions throughout the slaughter and cut-up process to reduce the populations and prevalence of Salmonella and Campylobacter. In majority of the cases, the Campylobacter populations on the broiler carcasses and chicken parts are relatively high compared with Salmonella and hence may not require use of a surrogate microorganism. Regardless, the correlation between Campylobacter and E. coli or Campylobacter and Salmonella populations on the chicken wings subjected to PAA exposure were poor (0.36 and 0.38, respectively), and hence, E. coli cannot be used as a surrogate microorganism for Campylobacter. Also, the data show that the efficacy of antimicrobial intervention(s) may be different for Salmonella and Campylobacter, and hence, the antimicrobial interventions should be evaluated for their efficacy against both Salmonella and Campylobacter in poultry operations.
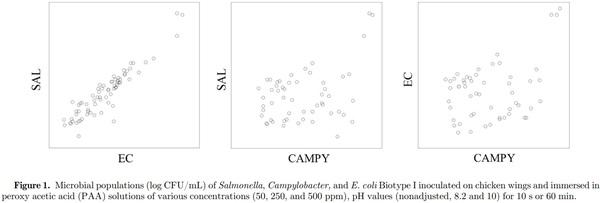
Concentrated PAA marketed for the food industry exists in equilibrium with H2O2 and acetic acid in water, and it is possible that the PAA and the H2O2 may exhibit antimicrobial activity through oxidation potential and the acetic acid through its ability as a weak acid. Considering the maximum PAA concentration used in the study (500 ppm), the concentrations of other components in such solution would be 500 ppm for H2O2 and between 1750 ppm (0.18%) and 5,000 ppm (0.5%) for acetic acid. Zhao and Doyle (2006) reported 2.0 log-reduction in Campylobacter populations by acetic acid (0.1% in water solution; pH 2.9) after 20 min of exposure and increasing the concentration to 0.5% (pH 2.8) resulted in > 7.2 log-reduction within 1 min of exposure. However, the Campylobacter reductions were lower (1.4 log10 CFU/g) on chicken wings with higher concentration of acetic acid (2%) and 15 s exposure time. It is possible the Campylobacter reductions would be minimal at lower acetic acid concentration of 0.5%.
Regardless, the mechanism of action of PAA solutions against bacteria could be from the oxidizing mechanism of PAA and H2O2 or the weak acid mechanism of acetic acid. Regarding the weak organic acid mode of action of acetic acid, these acids can penetrate the cell membrane in the undissociated state and dissociate upon encountering the high pH conditions in the cell cytoplasm. The hydrogen ions are actively expelled through the ATPases and active H+ pump systems. However, adjusting the pH of the PAA solution to its pKa (8.2) or higher will cause dissociation of the acetic acid, and hence, it cannot enter the cell, rendering the weak acid mode of action irrelevant. In addition, PAA can dissociate at these pH values, and it is possible that the dissociated PAA or the peroxyacetate ion still has the oxidation strength to denature proteins and lipids of microorganisms and affect the membrane function, resulting in cell death.
The PAA is highly unstable at alkaline pH and easily dissociates into hydrogen peroxide and acetic acid (Yuan et al., 1997). The authors stated that the consumption (depletion) of PAA may follow 3 routes: 1) spontaneous decomposition; 2) hydrolysis; and 3) transition metal catalyzed decomposition. The use of a chelating agent, such as 1- hydroxyethylidene-1,1-diphosphonic, can minimize the transition metal catalyzed decomposition. Further, the authors stated that in a pH range of 8.2 to 9.0, the PAA consumption is because of the spontaneous decomposition and hydrolysis. In this study, we used an alkaline pH of 8.2 (pKa of PAA) and 10, where decomposition and hydrolysis of PAA can occur. It is possible that these decomposition products still possess antimicrobial activity through their ability to denature proteins and lipids of microorganisms and affecting the membrane function, resulting in cell death.
Thus, PAA under alkaline pH environment does not rely on the typical mode of action of weak organic acids through perturbation of the internal pH of cells but rather the oxidizing activity. It is possible that even at the lower pH, the main mode of action of PAA can be attributed to its oxidizing activity, rather than the mode of action of weak organic acids as the concentrations required to cause bactericidal activity are far lower (in the ppm range; ca. 0.002%) than those required for the weak organic acids (> 0.5%).
Application of PAA as an immersion treatment was effective in reducing populations of Salmonella, Campylobacter, and E. coli on chicken wings. In general, longer treatment times (10 s vs. 60 min) and higher concentrations resulted in greater reductions in the microorganisms evaluated, although not statistically significant. Increasing the pH of the PAA solutions to 8.2 or 10 did not affect the antimicrobial activity of PAA. The 5 E. coli strains recognized by the USDA-FSIS for use in validation of HACCP systems can be used as surrogates for Salmonella in poultry processing. In plant, validation studies can be conducted using the surrogate organisms to evaluate the efficacy of antimicrobial interventions and the food safety system in poultry processing operations.














.jpg&w=3840&q=75)