High fidelity CRISPR/Cas9 increases precise monoallelic and biallelic editing events in primordial germ cells
Primordial germ cells (PGCs), the embryonic precursors of the sperm and egg, are used for the introduction of genetic modifications into avian genome. Introduction of small defined sequences using genome editing has not been demonstrated in bird species. Here, we compared oligonucleotide mediated HDR using wild type SpCas9 (SpCas9-WT) and high fidelity SpCas9-HF1 in PGCs and show that many loci in chicken PGCs can be precise edited using donors containing CRISPR/Cas9-blocking mutations positioned in the protospacer adjacent motif (PAM). However, targeting was more efficient using SpCas9-HF1 when mutations were introduced only into the gRNA target sequence. We subsequently employed an eGFP-to-BFP conversion assay, to directly compare HDR mediated by SpCas9-WT and SpCas9-HF1 and discovered that SpCas9-HF1 increases HDR while reducing INDEL formation. Furthermore, SpCas9-HF1 increases the frequency of single allele editing in comparison to SpCas9-WT. We used SpCas9-HF1 to demonstrate the introduction of monoallelic and biallelic point mutations into the FGF20 gene and generate clonal populations of edited PGCs with defined homozygous and heterozygous genotypes. Our results demonstrate the use of oligonucleotide donors and high fidelity CRISPR/Cas9 variants to perform precise genome editing with high efficiency in PGCs.
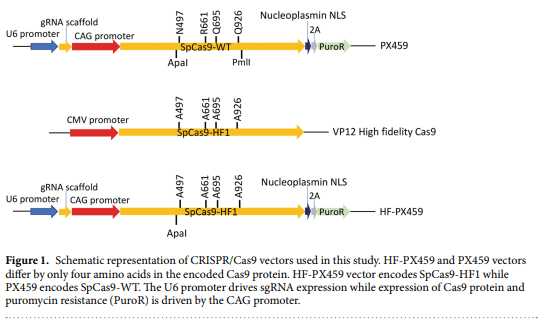
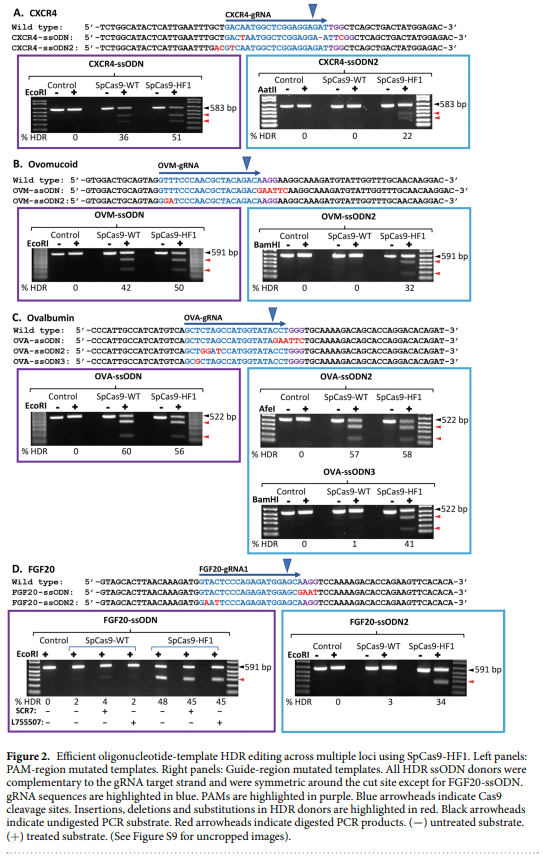
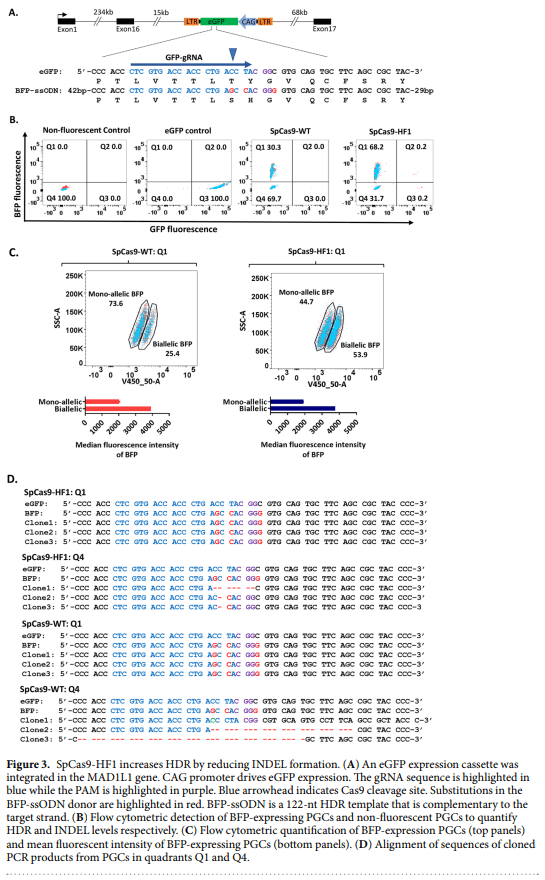
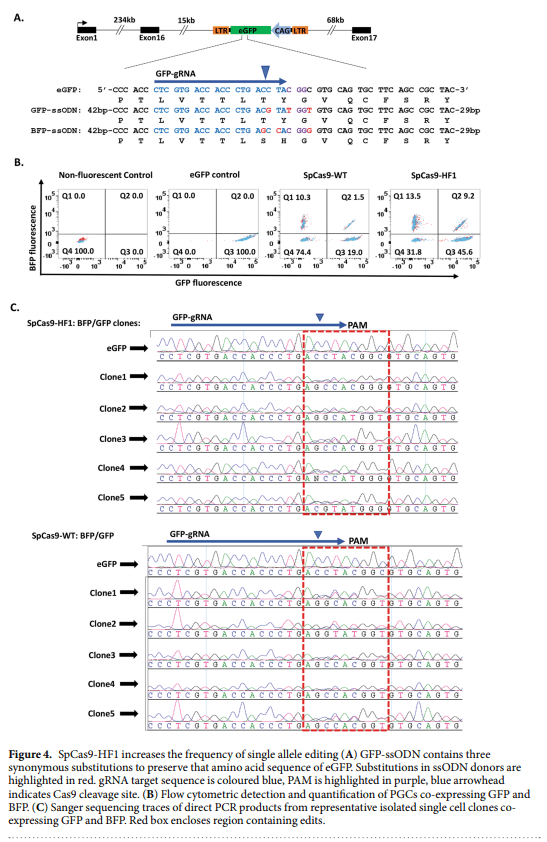
1. Lillico, S. G., McGrew, M. J., Sherman, A. & Sang, H. M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today 10, 191–196 (2005).
2. Scott, B. B., Velho, T. A., Sim, S. & Lois, C. Applications of avian transgenesis. ILAR J. 51, 353–361 (2010).
3. FAO. FAOSTAT- Livestock primary data. Available at: http://www.fao.org/faostat/en/#data/QL. (Accessed: 6th May 2018) (2016).
4. Brinster, R. L., Chen, H. Y., Trumbauer, M. E., Yagle, M. K. & Palmiter, R. D. Factors afecting the efciency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl. Acad. Sci. 82, 4438 LP–4442 (1985).
5. Tompson, S., Clarke, A. R., Pow, A. M., Hooper, M. L. & Melton, D. W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 56, 313–321 (1989).
6. Campbell, K. H. S., McWhir, J., Ritchie, W. A. & Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64 (1996).
7. Schnieke, A. E. et al. Human Factor IX Transgenic Sheep Produced by Transfer of Nuclei from Transfected Fetal Fibroblasts. Science (80-.). 278, 2130 LP–2133 (1997).
8. Sang, H. M. & Perry, M. M. Episomal replication of cloned DNA injected into the fertilised ovum of the hen, Gallus domesticus. Mol. Reprod. Dev. 1, 98–106 (1989).
9. van de Lavoir, M.-C. et al. High-grade transgenic somatic chimeras from chicken embryonic stem cells. Mech. Dev. 123, 31–41 (2006).
10. van de Lavoir, M.-C. et al. Germline transmission of genetically modifed primordial germ cells. Nature 441, 766–9 (2006).
11. Whyte, J. et al. FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal. Stem Cell Reports 5, 1171–1182 (2015).
12. Woodcock, M. E. M. E., Idoko-Akoh, A. & McGrew, M. J. M. J. Gene editing in birds takes fight. Mamm. Genome 28 (2017).
13. Schusser, B. et al. Immunoglobulin knockout chickens via efcient homologous recombination in primordial germ cells. Proc. Natl. Acad. Sci. USA 110, 20170–5 (2013).
14. Jasin, M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 12, 224–228 (1996).
15. Gaj, T., Gersbach, C. A. & Barbas, C. F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
16. Rothkamm, K., Krüger, I., Tompson, L. H., Kru, I. & Lo, M. Pathways of DNA Double-Strand Break Repair during the Mammalian Cell Cycle Pathways of DNA Double-Strand Break Repair during the Mammalian Cell Cycle. Mol. Cell. Biol. 23, 5706–5715 (2003).
17. Pardo, B., Gómez-González, B. & Aguilera, A. DNA Repair in Mammalian Cells. Cell. Mol. Life Sci. 66, 1039–1056 (2009).
18. Shrivastav, M., De Haro, L. P., Nickolof, J. A. & Haro, L. P. De. Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–47 (2008).
19. Chang, H. H. Y., Pannunzio, N. R., Adachi, N. & Lieber, M. R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 18, 495–506 (2017).
20. Li, X. & Heyer, W.-D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res 18, 99–113 (2008).
21. Orthwein, A. et al. A mechanism for the suppression of homologous recombination in G1 cells. Nature 528, 422–426 (2015).
22. Park, T. S., Lee, H. J., Kim, K. H., Kim, J.-S. & Han, J. Y. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl. Acad. Sci. USA 111, 1–6 (2014).
23. Oishi, I., Yoshii, K., Miyahara, D., Kagami, H. & Tagami, T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 6, 23980 (2016).
24. Dimitrov, L. et al. Germline gene editing in chickens by efcient crispr-mediated homologous recombination in primordial germ cells. PLoS One 11, e0154303 (2016).
25. Taylor, L. et al. Efcient TALEN-mediated gene targeting of chicken primordial germ cells. Development 144, 928 LP–934 (2017).
26. Armstrong, G. A. B. et al. Homology Directed Knockin of Point Mutations in the Zebrafsh tardbp and fus Genes in ALS Using the CRISPR/Cas9 System. PLoS One 11, e0150188 (2016).
27. Inui, M. et al. Rapid generation of mouse models with defned point mutations by the CRISPR/Cas9 system. Sci. Rep. 4, 5396 (2014).
28. Kistler, K. E., Vosshall, L. B. & Matthews, B. J. Genome Engineering with CRISPR-Cas9 in the Mosquito Aedes aegypti. Cell Rep. 11, 51–60 (2015).
29. Long, C. et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9–mediated editing of germline DNA. Science (80-.). 345, 1184 LP–1188 (2014).
30. Port, F., Chen, H.-M., Lee, T. & Bullock, S. L. Optimized CRISPR/Cas tools for efcient germline and somatic genome engineering in Drosophila Proc. Natl. Acad. Sci. 111, E2967 LP–E2976 (2014).
31. Wang, K. et al. Efcient Generation of Orthologous Point Mutations in Pigs via CRISPR-assisted ssODN-mediated Homologydirected Repair. Mol. Ter. - Nucleic Acids 5, e396 (2016).
32. Xiaoyang, Z. et al. Efcient Generation of Gene-Modifed Pigs Harboring Precise Orthologous Human Mutation via CRISPR/ Cas9-Induced Homology-Directed Repair in Zygotes. Hum. Mutat. 37, 110–118 (2015).
33. Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
34. Yu, C. et al. Small Molecules Enhance CRISPR Genome Editing in Pluripotent Stem Cells. Cell Stem Cell 16, 142–147 (2015).
35. Paquet, D. et al. Efcient introduction of specifc homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533, 125–129 (2016).
36. Yang, L. et al. Optimization of scarless human stem cell genome editing. Nucleic Acids Res. 41, 9049–9061 (2013).
37. Hwang, W. Y. et al. Efcient genome editing in zebrafsh using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–9 (2013).
38. Wang, L. et al. Enhancing Targeted Genomic DNA Editing in Chicken Cells Using the CRISPR/Cas9 System. PLoS One 12, e0169768 (2017).
39. Olsen, P. A., Solhaug, A., Booth, J. A., Gelazauskaite, M. & Krauss, S. Cellular responses to targeted genomic sequence modifcation using single-stranded oligonucleotides and zinc-fnger nucleases. DNA Repair (Amst). 8, 298–308 (2009).
40. Rios, X. et al. Stable Gene Targeting in Human Cells Using Single-Strand Oligonucleotides with Modifed Bases. PLoS One 7, e36697 (2012).
41. Paix, A. et al. Precision genome editing using synthesis-dependent repair of Cas9-induced DNA breaks. Proc. Natl. Acad. Sci. 114, E10745–E10754 (2017).
42. Bialk, P. et al. Analyses of point mutation repair and allelic heterogeneity generated by CRISPR/Cas9 and single-stranded DNAoligonucleotides. Sci. Rep. https://doi.org/10.1038/srep32681 (2016).
43. Merkle, F. T. et al. Efcient CRISPR-Cas9-Mediated Generation of Knockin Human Pluripotent Stem Cells Lacking Undesired Mutations at the Targeted Locus. Cell Rep. https://doi.org/10.1016/j.celrep.2015.04.007 (2015).
44. Chen, J. S. et al. Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 550, 407 (2017).
45. Kleinstiver, B. P. et al. High-fdelity CRISPR–Cas9 nucleases with no detectable genome-wide of-target efects. Nature 529, 490–495 (2016).
46. Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specifcity. Science 351, 84–88 (2016).
47. Glaser, A., McColl, B. & Vadolas, J. GFP to BFP Conversion: A Versatile Assay for the Quantifcation of CRISPR/Cas9-mediated Genome Editing. Mol. Ter. - Nucleic Acids 5 (2017).
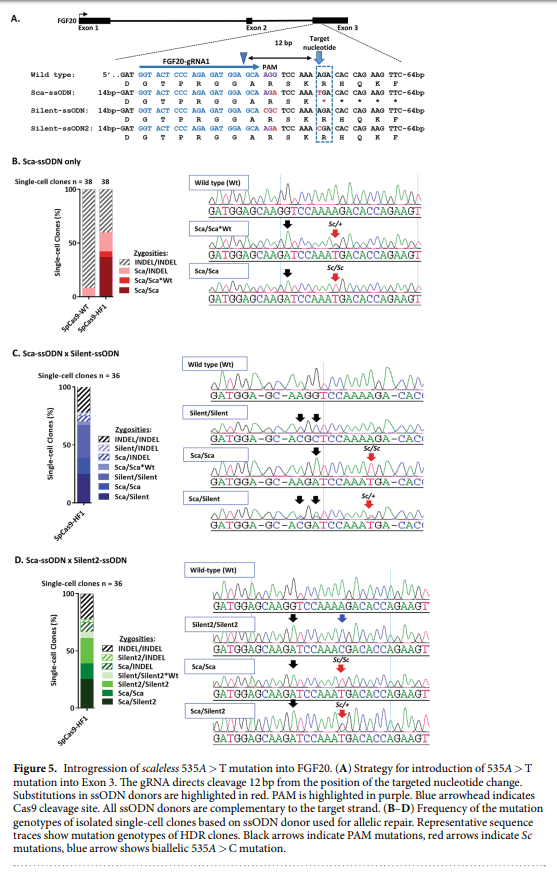
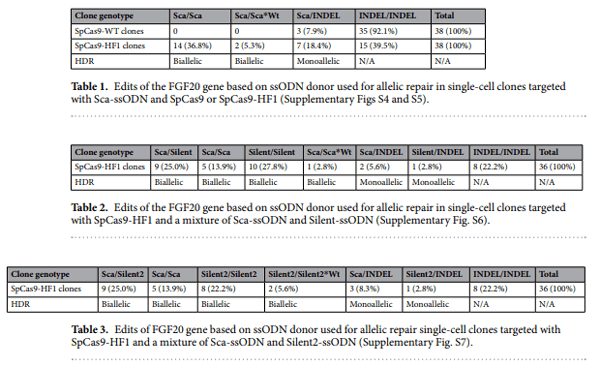
48. Wells, K. L. et al. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genomics 13, 257 (2012).
49. Cahaner, A. et al. Efects of the Genetically Reduced Feather Coverage in Naked Neck and Featherless Broilers on Teir Performance Under Hot Conditions. Poult. Sci. 87, 2517–2527 (2008).
50. Azoulay, Y. et al. Te viability and performance under hot conditions of featherless broilers versus fully feathered broilers. Poult Sci 90, 19–29 (2011).
51. Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife 3, 1–13 (2014).
52. Oji, A. et al. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci. Rep. 6, 31666 (2016).
53. Maruyama, T. et al. Increasing the efciency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33, 538–42 (2015).
54. Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marrafni, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
55. Zheng, T. et al. Profling single-guide RNA specifcity reveals a mismatch sensitive core sequence. Sci. Rep. 7 (2017).
56. Semenova, E. et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc. Natl. Acad. Sci. 108, 10098–10103 (2011).
57. Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (New York, N.Y.) 337, 816–21 (2012).
58. Hsu, P. D. et al. DNA targeting specifcity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
59. Fu, Y. et al. High-frequency of-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31, 822–826 (2013).
60. Cho, S. W. et al. Analysis of of-target efects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 24, 132–141 (2014).
61. Gao, Z., Harwig, A., Berkhout, B. & Herrera-Carrillo, E. Mutation of nucleotides around the+1 position of type 3 polymerase III promoters: Te efect on transcriptional activity and start site usage. Transcription 8, 275–287 (2017).
62. Howden, S. E. et al. A Cas9 Variant for Efcient Generation of Indel-Free Knockin or Gene-Corrected Human Pluripotent Stem Cells. Stem Cell Reports, https://doi.org/10.1016/j.stemcr.2016.07.001 (2016).
63. Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168–e168 (2014).
64. Wang, H. et al. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell 153, 910–918 (2013).
65. Liang, X., Potter, J., Kumar, S., Ravinder, N. & Chesnut, J. D. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. J. Biotechnol. 241, 136–146 (2017).
66. Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L. & Corn, J. E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donorDNA. Nat Biotech 34, 339–344 (2016).
67. Sentmanat, M. F., Peters, S. T., Florian, C. P., Connelly, J. P. & Pruett-Miller, S. M. A Survey of Validation Strategies for CRISPR-Cas9 Editing. Sci. Rep. 8, 888 (2018).
68. Knight, S. C. et al. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science (80-.). 350, 823 LP–826 (2015).
69. Chen, X. et al. Probing the impact of chromatin conformation on genome editing tools. Nucleic Acids Res. 44, 6482–6492 (2016).
70. Horlbeck, M. A. et al. Nucleosomes impede Cas9 access to DNA in vivo and in vitro. Elife 5, e12677 (2016).
71. Kato-Inui, T., Takahashi, G., Hsu, S. & Miyaoka, Y. Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPRassociated protein 9 with improved proof-reading enhances homology-directed repair. Nucleic Acids Res. gky264-gky264 (2018).
72. Zhang, D. et al. Perfectly matched 20-nucleotide guide RNA sequences enable robust genome editing using high-fdelity SpCas9 nucleases. Genome Biol. 18, 191 (2017).
73. Kim, S., Bae, T., Hwang, J. & Kim, J.-S. Rescue of high-specifcity Cas9 variants using sgRNAs with matched 5′ nucleotides. Genome Biol. 18, 218 (2017).
74. Kleinstiver, B. P. et al. Engineered CRISPR-Cas9 nucleases with altered PAM specifcities. Nature 523, 481 (2015).
75. Zhang, Y. et al. Comparison of non-canonical PAMs for CRISPR/Cas9-mediated DNA cleavage in human cells. Sci. Rep. 4, 5405 (2014).
76. Song, J. et al. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efciency. Nat. Commun. 7, 10548 (2016).
77. Hou, M. et al. Doxorubicin Induces Apoptosis in Germ Line Stem Cells in the Immature Rat Testis and Amifostine Cannot Protect against Tis Cytotoxicity. Cancer Res. 65, 9999 LP–10005 (2005).
78. Olsen, A.-K., Lindeman, B., Wiger, R., Duale, N. & Brunborg, G. How do male germ cells handle DNA damage? Toxicol. Appl. Pharmacol. 207, 521–531 (2005).
79. Liu, G. et al. Efect of Low-Level Radiation on the Death of Male Germ Cells. Radiat. Res. 165, 379–389 (2006).
80. Xu, G., Vogel, K. S., McMahan, C. A., Herbert, D. C. & Walter, C. A. BAX and Tumor Suppressor TRP53 Are Important in Regulating Mutagenesis in Spermatogenic Cells in Mice. Biol. Reprod. 83, 979–987 (2010).
81. Habas, K., Anderson, D. & Brinkworth, M. H. Germ cell responses to doxorubicin exposure in vitro. Toxicol. Lett. 265, 70–76 (2017).
82. Kim, S., Kim, D., Cho, S. W., Kim, J. & Kim, J.-S. Highly efcient RNA-guided genome editing in human cells via delivery of purifed Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
83. Ihry, R. J. et al. p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells. Nat. Med. https://doi.org/10.1038/s41591- 018-0050-6 (2018).
84. Haapaniemi, E., Botla, S., Persson, J., Schmierer, B. & Taipale, J. CRISPR–Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. https://doi.org/10.1038/s41591-018-0049-z (2018).
85. Fridman, J. S. & Lowe, S. W. Control of apoptosis by p53. Oncogene 22, 9030 (2003).
86. Labun, K., Montague, T. G., Gagnon, J. A., Tyme, S. B. & Valen, E. CHOPCHOPv2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44, W272–W276 (2016).
87. Montague, T. G., Cruz, J. M., Gagnon, J. A., Church, G. M. & Valen, E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401–W407 (2014).
88. McGrew, M. J. et al. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development 135, 2289 (2008).
89. Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 195, 231–272 (1992).
90. Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115–e115 (2012).
91. Koressaar, T. & Remm, M. Enhancements and modifcations of primer design program Primer3. Bioinformatics 23, 1289–1291 (2007).
92. Guschin, D. Y. et al. In Engineered Zinc Finger Proteins: Methods and Protocols(eds Mackay, J. P. & Segal, D. J.) 247–256, https://doi. org/10.1007/978-1-60761-753-2_15 (Humana Press, 2010).










(25minEng).jpg&w=3840&q=75)