Following response to Sel-Plex® and other organic minerals through the broiler breeder maze: case studies in Brazil
Years of genetic selection for growth have created a bird unfit for self-regulation of feed intake due to numerous problems linked to over-consumption. Renema and Robinson (2004) mentioned that under current conditions, the negative relationship between excess nutrient intake and reproduction must be altered by management strategies, including feeding. Because growth rate and muscle mass of broiler stocks have increased so much over the past 50 years, both ‘normal’ feed intake and ‘appropriate’ feed intake of broiler parent stock needs to be redefined.
There are several fields to be investigated to improve broiler breeder performance, mineral nutrition being among them. Most mineral requirements were established many years ago. As mentioned above, the breeder has changed and therefore nutritional requirements need to be updated. This paper examines the improvement in breeder performance when organic minerals, such as organic selenium (Se) alone or in combination with organic and inorganic manganese (Mn) and zinc (Zn) are used.
Absorption and metabolic roles of selenium, zinc and manganese
Selenium is often found in association with sulfur in inorganic and organic compounds. Common forms of Se are selenic acid, selenates and selenites. Some plants and microorganisms have been shown to be able to replace the sulfur in cysteine and methionine with Se, thereby producing selenocysteine and selenomethionine (Leeson and Summers, 2001).
Selenomethionine is absorbed from the digestive tract by an active transport mechanism similar to that involved in the transport of methionine, while inorganic selenite and selenocysteine are not actively transported. Once absorbed, inorganic Se binds to Se-binding proteins. Selenium intake in excess of that bound by these proteins appears to be methylated in preparation for excretion. This chemical reaction takes place in two steps: either the formation of dimethyl selenide, or its further conversion to trimethyl selenonium ion, which is water soluble and represents the normal urinary excretory product of a moderate excessive intake of the element. If Se supplies exceed the body’s ability to convert it to a chemical selenonium ion, then dimethyl selenide, a volatile compound, is excreted via the lungs, imparting a garlic-like odor (Leeson and Summers, 2001).
Selenium can be supplied as an inorganic salt, such as sodium selenite or as part of organic molecules, such as those synthesized by Se-enriched yeast. However, the utilization efficiency of these two sources of Se is different. Inorganic Se is retained at a much lower concentration in muscle than organic sources (Mahan and Parrett, 1996). Furthermore, several investigators have demonstrated that organic Se from Sel-Plex® Seyeast is more effectively transferred from the diet of broiler breeders to the egg (Cantor, 1997; Surai, 2000; Paton et al., 2002) and to the tissues (Surai, 2000; Paton et al., 2002) of day-old chicks.
Selenium is an essential component of glutathione peroxidase (GSH-Px). This enzyme aids in protecting cellular contents and subcellular membranes from oxidative damage. Vitamin E is a specific lipid soluble antioxidant in the membrane, whereas Se functions as a component of GSH-Px that destroys peroxides before they attack cellular and organelle membranes. Besides this most well known function of Se, other metabolic roles are important to broilers: 1) Se plays a role in RNA, since it can be incorporated into purines and pyrimidine bases, 2) it may have a specific role in prostaglandin synthesis and essential fatty acid metabolism, 3) both vitamin E and Se are needed for adequate immune responses and 4) Se is important for activation of thyroid hormones. (For further information, see Jacques, 2001).
A Se-deficient diet reduces hatchability (Jensen, 1968). Chicks that hatch are weak and have gizzard muscle myopathy, and are prostrate with their legs extended backward and curved upward. Arnold et al. (1974) reported a significant effect of Se on hatchability, however, the data were not definitive.
Zinc (Zn) is a transitional metal that exists in a unique oxidative state, which allows it to function as a structural element in enzymatic proteins or as a co-factor for enzyme activity (Pedrieri and Cinti, 2003). Zinc is a functional component of several enzyme systems, including those involved in growth, digestion and respiration. Among the enzymes requiring Zn are carbonic anhydrase, alcohol dehydrogenase, alkaline phosphatase, cytosolic superoxide dismutase, tymidine kinase and DNA-dependent RNA polymerases (Maas, 1999).
The role of Zn in embryo development has been examined by Wilson (1997). Low levels of Zn are necessary in the breeder diet to obtain normal hatchability. A Zn deficiency in the breeder diet results in decreased hatchability, increased embryonic mortality and impaired development of the skeleton and feathers. Numerous abnormalities have been described in embryos of Zn-deficient eggs.
Manganese is a functional component of many enzyme systems, including those involved with carbohydrate, protein and lipid metabolism. Some of these enzymes are isocitrate dehydrogenase, Mn-specific glycosyl glycosyltransferase, mitochondrial superoxide dismutase and pyruvate carboxylase. In addition, Mn is a component of the matrix bone, therefore required for normal bone structure (McDowell, 2003).
Wilson (1997) examined the role of Mn on embryo development and concluded that the deficiency of this mineral results in reduced hatchability and embryonic abnormalities. Microscopic differences in the structure of bones are present. Ataxia in the form of tetanic spasm has been described in Mn-deficient embryos. The breeder may be able to tolerate a lack of Mn supplementation in the diet for several weeks with no adverse effects on hatchability.
Brazilian background in poultry and the importance of trials with organic minerals
Brazil’s primary poultry producing region is in the southernmost states of São Paolo, Paraná, Santa Catarina and Rio Grande do Sul (Figure 1). In a recent publication (Poultry International, Vol. 44 (1):4-8, 2005) it was stated that Brazil is one of the most important countries in broiler production. In the year 2004, Brazil took over from the US as the leading exporter of broiler meat. Furthermore, the most recent forecasts coming out of the USDA point to Brazil holding on to this position in 2005.
Brazilian broiler meat exports in 2005 are projected to increase by 10%, fuelled by competitive pricing, market promotion efforts, favorable exchange rates and avian influenza-related import bans on major competitors. Brazil’s market strategy has been to increase valued-added poultry meat (such as high-end cuts and processed broilers) and the bulk of the business is to the top import markets. Combined with relatively low feed grain costs, relatively low labor costs, and steadily larger economies of scale, Brazil’s production costs for whole eviscerated chicken are estimated to be the lowest of any major supplier. If forecasts by the USDA Foreign Agricultural Service prove correct, then in 2005 Brazil will indeed retain its position as the leading exporter of broiler meat.
It is important to point out that the Brazilian poultry industry is ever eager to adopt new technologies in all areas, nutrition being one of them. One of the most fascinating branches of nutrition is the use of organic minerals. Examining several Brazilian field trials, Rutz et al. (2003) have indicated that the addition of Sel- Plex® and other organic minerals to commercial breeder diets increased the number of chicks/hen housed from 0.945 to 3.62. Those results were corroborated under US field conditions (Edens, 2002) and in experiments conducted in universities (Renema, 2004).
A series of field trials using organic minerals was conducted in several Brazilian poultry companies. Broiler breeder hens and roosters received diets supplemented with Sel-Plex® in combination with Bioplex™ Zn and Bioplex™ Mn. The starting and the ending ages of supplementation varied throughout the studies. General descriptions are given below.
COMMERCIAL EXPERIENCE WITH ORGANIC MINERALS IN BREEDER DIETS
Trial 1: Cobb breeders in Paraná
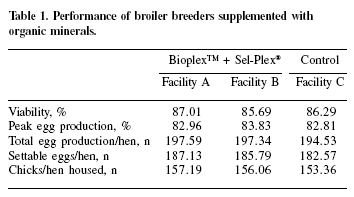
An integrator farm in the state of Paraná has around 3 million broiler breeders. From those, a total of 15,900 Cobb breeder hens were equally divided into three groups. The female:male ratio was 10:1. The control flock (5,300 hens) was fed a corn-soybean meal basal diet containing 0.3 ppm Se as sodium selenite, 100 ppm Zn (from an inorganic source) and 100 ppm Mn (from an inorganic source). The test flocks (5300 hens each, allocated in two different facilities) received the control diet with the addition of 0.3 ppm Se from Sel-Plex®, 30 ppm Zn from Bioplex™ Zn and 30 ppm Mn from Bioplex™ Mn. The diets were offered from 23 to 68 weeks of age. Viability, peak egg production, total egg production, settable eggs, chicks per hen housed were evaluated; and an extra 3.26 extra chicks/hen were noted in breeders given the organic mineral-supplemented diets (Table 1).
A second trial, conducted with the same integrator as in Trial 1, involved a total of 30,000 Ross breeder hens plus 3000 males. The birds were distributed in six facilities. In three of those facilities, the birds were fed a corn-soybean meal basal diet containing 0.3 ppm Se as sodium selenite, 100 ppm Zn (from an inorganic source) and 100 ppm Mn (from an inorganic source). The test group in the three remaining facilities received the same basal diet with the addition of 0.2 ppm Se as Sel-Plex®, 30 pm Zn as Bioplex™ Zn and 30 ppm Mn as Bioplex™ Mn. The diets were offered from 23 to 66 weeks of age. In this study supplementation of the organic minerals resulted in an extra 2.91 chicks per hen (Table 2).

Trial 3: Hybro breeders in São Paulo
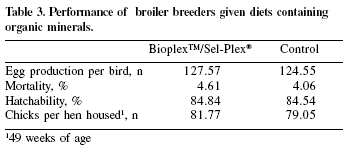
This trial was conducted at an integrated farm in the state of São Paulo. A total of 18,000 Hybro breeders were divided in two groups and allocated in two farms (9,000 birds each). The control group received a cornsoybean meal basal diet containing 0.3 ppm Se (as sodium selenite), 100 ppm Zn (from an inorganic source) and 100 ppm Mn (from an inorganic source). The test group received the same basal diet with the addition of 0.2 ppm Se as Sel-Plex®, 30 pm Zn as Bioplex™ Zn and 30 ppm Mn as Bioplex™ Mn. The diets were offered from 23 to 52 weeks of age. Improvements in mortality, total egg production and hatchability resulted in an added 2.72 chicks per hen housed at 49 weeks of age (Table 3).
Trial 4: Cobb breeders in São Paulo
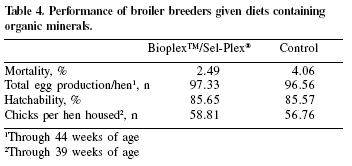
This trial was conducted at an integrated farm in the state of São Paulo. A total of 19,000 23-44 week old Cobb breeders were divided in two groups and allocated in two farms (9,500 birds each). The control group received a corn-soybean meal basal diet containing 0.3 ppm Se (as sodium selenite), 100 ppm Zn (from an inorganic source) and 100 ppm Mn (from an inorganic source). The test group received the basal with the addition of 0.2 ppm Se as Sel-Plex®, 30 pm Zn as Bioplex™ Zn and 30 ppm Mn as Bioplex™ Mn. The diets were offered from 23 to 44 weeks of age. As in the previous trials, changes in mortality, total egg production and hatchability resulted in a total of 2.05 extra chicks/hen when diets were supplemented with organic minerals (Table 4).
Discussion and further comments on the use of organic minerals for broiler breeder hens
Several field trials were conducted in the Brazilian poultry industry to investigate the performance of broiler breeder hens fed diets containing Sel-Plex®, Bioplex™ Zn and Bioplex™ Mn. Since there was no replication in those commercial feed trials, statistical analysis was not possible, but a numerical and commercially important advantage was consistently demonstrated for organic minerals.
Addition of Sel-Plex® and other organic minerals to breeder diets starting at 23 weeks of age improved reproductive performance of the broiler breeders (Tables 1 to 4). Overall reproductive efficiency is determined by interaction of four variables: ovulation rate x male fertility, and female fertility x hatch x post-hatch survival. To optimize total chick production under given management conditions, the manager must understand how these variable are related and apply this understanding in a practical focus on the hen, rooster or their combination (Hammerstedt, 1999).
OVULATION AND EGG PRODUCTION
All trials showed that breeder hens given organic mineral supplements had higher egg production (Tables 1 to 4). Broiler breeders have generally been considered to be poor layers because after they reach peak production, performance declines rapidly compared with commercial egg layer strains. This poor performance is attributed to their tendency to become overweight after peak production.
In spite of that, several studies with layers (Pan et al., 2004 and Xavier et al., 2004) and with broiler breeders (Edens, 2002; Rutz et al., 2003) have indicated that use of Sel-Plex® alone or in combination with other organic minerals improves egg production. The reason for the improvement in egg production due to organic Se supplementation is not clear, but may be related to its major component, selenomethionine, which can be stored in the body. Cave (1984) and Brake et al. (1985) attributed the increase in egg production in their trials to an increase in protein deposition in the body.
On the other hand, egg production is an estimation of the rate of ovulation and oviposition (Etches, 1996). It may be postulated that the increase in egg production could have resulted from an improvement in ovulation. This improvement in egg production may indicate more appropriate follicle maturation. This condition indicates that follicle receptors (integral proteins) for gonadotropins are better synthesized. Antioxidant protection from GSH-Px and the role of Zn in protein synthesis could have contributed to the synthesis and release of LH and FSH hormones and synthesis of their receptors.
SETTABLE EGGS
The number of settable eggs per hen housed was improved (Tables 1 and 2 ) by adding organic minerals to the breeder diets. Similar results were reported using Sel-Plex® in field trials conducted in the US (Edens, 2002) and under controlled experimental conditions (Renema, 2004). In addition to higher bioavailability, it can be postulated that Sel-Plex® organic Se may aid the absorption and availability of other nutrients (Surai and Sparks, 2000; Pan et al., 2004), which can be deposited in the developing follicle, increasing its size.
FERTILITY
Number of chicks hatched per hen housed provides a measure of egg production, fertility and hatchability and is a useful yardstick for the economic efficiency of the parent flocks and the hatchery (Etches, 1996). Hammerstedt (1999) indicated that fertility success depends of the potential of eggs to be fertilized (female) and sperm with high fertilization potential (male), production of embryos (males and females) and high quality chicks during the first days after hatching. It is assumed that if a hen is capable of producing an egg, and if viable sperm are available, fertility will occur (Leeson and Summers, 1997), although it has been shown that female fertility can be influenced by energy and protein feeding programs (McDaniel et al., 1981) and by dietary organic minerals.
The increased number of chicks hatched per hen housed in response to dietary organic minerals ranged in commercial trials in Brazil from 2.05 (Table 4) to 3.26 (Table 1). These results are corroborated by other studies, which showed improvements ranging from 1.2 (Edens, 2002) to 3.62 (Rutz et al., 2003) chicks/hen housed in favor of breeders fed Sel-Plex® organic Se alone or with other organic minerals. Although it is stated that the Se requirement of poultry under physiological conditions is thought to be quite low, varying from 0.06 (laying hen) up to 0.2 ppm (turkey, duck), the improvement in the number of chicks observed by adding organic minerals can be explained by various stresses observed under commercial conditions, which certainly bring about an increase in demand for Se and other stress/ antioxidant-related minerals (Surai, 2002).
Bekhtina (1968) showed that while only one spermatozoa unites with the ovum to form the zygote, many spermatozoa may play a role in the early development of the embryo. Breeding efficiency is more directly quantifiable as the number of spermatozoa that become associated with the perivitelline membrane at or around the time of fertilization (Bramwell et al., 1995), which is representative of the number of spermatozoa stored in the oviduct (Wishart and Staines, 1999). In broiler breeder flocks, the median number of points of hydrolysis produced by spermatozoa in the inner perivitelline layer over the germinal disc of eggs is related to flock fertility (Hazary et al., 2000). Renema (2004) has demonstrated that breeder hens fed Sel-Plex® showed a higher number of sperm holes at the site of fertilization in the perivitelline membrane, as compared to hens fed inorganic Se. He attributed this effect to changes in the oviduct environment, such as reduction of free radicals within the sperm host glands, due to improvement in GSH-Px activity (Surai, 2000).
In order to achieve the highest level of fertility and hatch of fertile eggs, an adequate number of active males producing high quality semen should be maintained in the breeder house at all times (Eslick and McDaniel, 1992). Feeding heavy roosters diets containing sodium selenite or Sel-Plex®, Silva (personal communication)
indicated that Sel-Plex®-fed males had a clear trend for decreased sperm tail mid-piece abnormalities and a significant increase in the number of sperm that attach to the perivitelline membrane.
Under similar conditions, Edens (2002) has shown that Sel-Plex® Se-fed roosters had higher sperm quality as compared to those fed diets containing no additional or additional inorganic Se. It is important to point out that Se, as a component of GSH-Px, helps protect lipid-rich membranes of sperm. Higher trace mineral status through use of organic forms can potentially affect several factors related to both males and females that interfere in the perforation of the perivitelline layer. Among them, an increase in densities of the sperm receptor, an improvement of the acrosome reaction or an increased susceptibility of the perivitelline layer to hydrolysis (Bramwell and Howarth, 1992; Kuroki and Mori, 1997).
Furthermore, selection programs based on growth efficiency and meat yields have successfully been used in the broiler industry (Kerr, 1998). However, the existence of negative phenotypic and genetic correlations between growth-related traits and reproductive performance has led to increased fertility problems in both male and female lines of fast growing birds (Brillard, 2004).
For example, in pedigree lines of commercial breeder stocks selected for high growth rate, Reddy and Sadjadi (1990) observed negative correlations between body weight and semen quality traits (sperm motility, sperm density), such correlations varying from line to line. Furthermore, these authors predicted an approximately 0.5%/year decrease in fertility performance of these flocks in the absence of a balanced breeding approach to counter this trait. Due to improvement of sperm quality traits with the use of Sel-Plex®, as above mentioned, it can be postulated that this product can be used to alleviate and counteract the adverse effects brought about by the genetic selection programs.
HATCHABILITY
The factors that contribute to hatchability are egg fertility and embryonic mortality. Prolonged and abnormal egg storage conditions, broiler breeder age and diet influence fertility, subsequent embryogenesis and hatchability, which are the major factors to be considered in performance of broiler breeders. Intermittent periods of infertility identified in naturally mated broiler breeder hens have been reported (Fontana et al., 1992; Barbato, 1999). It is important to differentiate the extent to which changes in overall hatchability are due to changes in fertility. The use of Sel-Plex® improved hatchability (Table 4). These results agree with those observed by other investigators (Edens, 2002; Rutz et al., 2003; Renema, 2004).
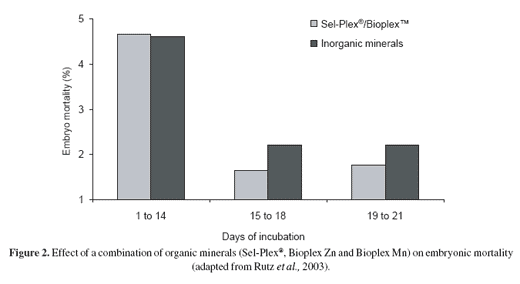
An egg that fails to hatch is infertile or it is fertile but the embryo suffers mortality prior to or during incubation. Two out of the three weeks of incubation are characterized by an increase in mortality. During the first week of embryonic development, mortality occurs due to vascular malformation (Etches, 1996) and during the third mortality is due to changes in nourishment and in the respiratory system (Payne, 1919). The use of organic minerals (Sel-Plex®, Bioplex™ Zn and Bioplex™ Mn) has been shown to prevent or at least alleviate embryonic mortality during late embryonic development (Figure 2).
Carbohydrate and protein metabolism predominate during the first two weeks of incubation, with the last seven days of incubation being noted as a period of intense lipid metabolism and rapid embryo growth in which some 80% of the entire lipid content is mobilized and absorbed into the embryonic tissue (Romanoff, 1960; Noble and Cocchi, 1990). Fatty acid oxidation is the primary source of energy and metabolic water for developing embryos (Rahn and Paganelli, 1991). Part of this lipid material is composed of unsaturated fatty acids, which are prone to peroxidation (Surai, 2002).
The beneficial effect of Se during embryo development was verified by Paton et al. (2002). First of all, total egg Se content is higher when Se is provide to the hen in organic form. Organic Se as Sel-Plex® also enhanced the antioxidant profile of the embryo (Surai and Sparks, 2000). Therefore, incorporation of Se (as a component of GSH-Px) by the body of the chick brings about protection against oxidation reactions.
In addition, there is an increase in conversion of linoleic acid to arachidonic acid, an important component of cell membrane phospholipids. That fatty acid is unsaturated and therefore needs protection from oxidizing reactions. Recently, Pappas et al. (2004) indicated that dietary fish oil (or PUFAs) may affect Se metabolism and deposition in the tissues rather than Se absorption from the diet. Fish oil may therefore influence uptake mechanisms for Se in the embryo.
Selenium status is also critical to hematopoiesis (Paton et al., 2002). On day 12 of incubation, the spleen begins to function as a hematopoietic organ and produces primitive lymphoid cells that differentiate into leucocytes and erythrocytes. The development of erythrocytes would require additional synthesis of GSH-Px. Finally, Paton and coworkers mentioned that the adenohypophyseal-thyroid axis (embryonic thyroid) of the chick becomes functional on day 11.5 of incubation and the thyroid of the chick begins to convert thyroxin to triiodothyronine, the active form of thyroid hormone. This conversion depends on deiodinase enzymes, which are Se-dependent.
The added Bioplex™ organic Zn and Mn may have helped in embryo development and survival during the late part of incubation. Vieira and Moran (1998) compared mineral content of 27 and 62-week old broiler breeder hens and indicated that there is a reduction in yolk Zn and Mn content in older hens. Feeding layer (Guo et al., 2002) and broiler breeder (Hudson et al., 2004a) hens diets containing organic Zn increased egg Zn content. In addition, Badawy et al. (1987) found a positive relationship between Zn concentration of eggs and hatchability. Therefore, Hudson et al. (2004a) hypothesized that inadequate transmission of Zn from the hen to the hatching egg is likely responsible for low hatchability and poor chick quality when Zn intake is insufficient.
According to Hudson et al. (2004a), organic and inorganic Zn in combination increase the number of settable eggs per hen housed, and thus the total number of chicks produced, but not fertility or hatchability. The authors proposed two mechanisms of action for better performance due to the combination of organic and inorganic Zn sources as opposed to either fed as the sole source of dietary Zn. First, absorption of Zn and Zn status of the bird may be improved.
Supplementing a diet with both organic and inorganic sources of Zn may involve more absorption sites or transporters in the intestine, increasing Zn retention and enhancing performance. In addition, chelation of Zn by phytic acid in the gastrointestinal tract may be reduced and absorption is likely increased when organic Zn sources are provided in the diet. A second possible mode of action may involve effects of dietary Zn source on Zn metabolism. Dietary Zn from organic sources may be absorbed intact and function differently than Zn from inorganic source after being absorbed. Suso and Edwards (1972) suggested that Zn may be transferred to enzymes as a metalloligand complex, and the ligand could influence enzyme activity.
Zinc is also involved in immune status (Leeson and Summers, 2001). Hudson et al. (2004a) have shown that organic Zn enhanced the immune status of hens, indicating that disease resistance or transmission of antibodies to progeny may be enhanced when broiler breeder hens consume organic Zn. However, in their study that effect was not translated into lower embryonic mortality. In addition, supplemental Zn from an organic or inorganic source in breeder hen diets did not influence chick physiology at hatching (Hudson et al., 2004b).
PERFORMANCE OF PROGENY
According to Lopez and Leeson (1994), the biological function of the broiler breeder is to produce a viable embryo with the features required to produce a broiler chicken. A fertile egg will provide a closed environment within which all nutritional needs of the embryo must be met, with the notable exception of gaseous change. Therefore, the physiological condition of the broiler breeder hen and the egg are related to normal embryo development.
A high positive relationship between egg weight and chick size has been reported by several workers (Shanawany, 1987; Pinchasov, 1991) and chick weight is usually 62-76% of egg weight (Lopez and Leeson, 1994). Chick weight at hatch affects subsequent broiler growth. It has been reported that a 1 g difference in egg weight results in 0.5 g in chick size, which subsequently results in an approximate 5 g difference in body weight at 42 days of age (Leeson and Summers, 2000). Higher broiler weight at slaughter is the main goal of the farmer. Egg size increases with breeder age, so it would be expected that bird age will also affect chick weight.
Dietary manipulation of certain nutrients during the latter parts of the growing period and the early stages of the egg production cycle has the potential to increase early egg size. Previous investigations have shown that early egg size can be increased by increasing dietary protein during the early stages of egg production (Keshavarz, 1995). Changes in protein and amino acid content of the diet primarily affect the deposition of albumen (Etches, 1996; Joseph et al., 2000). The benefit of higher dietary protein on early egg size can be further increased by supplemental fat in isocaloric diets (Keshavarz and Nakajima, 1995). It is important to point out that changes in the consumption of linoleic acid alter yolk formation (Etches, 1996).
Replacement of inorganic Se with Sel-Plex® has been shown to increase both the yolk and albumen weight (Pan et al., 2004; Xavier et al., 2004) as well as the dry matter content of the yolk (Lara, personal communication). Because chick size is positively correlated with egg size, it seems reasonable to assume that changes in the diet that influence egg weight will also influence the size of the chick at hatch and subsequent performance. Surai (2000) observed higher GSH-Px activity in chicks from breeders fed Sel-Plex® than in those fed sodium selenite. This fact certainly explains the lower culling and mortality of chicks from hens fed Sel-Plex® (Lanning et al., 2000). Furthermore, these data are corroborated by results of Rebel et al. (2004), who reported that additional vitamins and trace minerals (Se, copper and Zn) in breeder diets can stimulate the immune system of chicks.
ALBUMEN CONSISTENCY AND INCUBATION: POSSIBLE ROLE OF SEL-PLEX® FOR DELAYED INCUBATION?
After oviposition, there is a rise in albumen pH with storage time, and hen age is associated with a decrease in albumen height and viscosity. Albumen liquefaction probably facilitates the movement of nutrients from the albumen to the blastoderm (Brake et al., 1997) and may reduce resistance to gaseous diffusion (Meuer and Baumann, 1988). This explains why short-term storage may have beneficial effects on hatchability of eggs from young flocks (Brake, 1996), as these flocks generally lay eggs that have albumen of good quality (density) that is fairly resistant to degradation. However, extended periods of egg storage allow the albumen to degrade excessively. This degradation causes the blastoderm to move into close proximity to the egg shell, so that early embryonic mortality results from dehydration during the early stages of incubation (Brake et al., 1993). Lapão et al. (1999) have shown that in older breeder flocks, the decline in hatchability starts 1 day after lay, possibly due to deterioration in egg albumen quality.
The detrimental effect of long-term storage is more pronounced in eggs from older breeder flocks (Kirk et al., 1980), which is a result of lower albumen quality at oviposition and a consequent increased rate of decline during storage (Hurnik et al., 1978). Elibol and Brake (2004) observed that fertile egg hatchability was higher from a broiler breeder flock at 29 weeks of age than at 68 weeks of age because of decreased mortality at all stages of embryo development. Recently, Tona et al. (2004) confirmed those observations and indicated that fresh eggs from young breeders had better albumen quality, hatched better, and produced a higher percentage of high quality chicks, although with lower weights at hatch, compared with older breeders but showed greater post-hatch growth rate.
The low egg albumen quality effect has been shown to be alleviated by adding Sel-Plex® to the breeder diets in several studies. Examining the effect of Sel-Plex® on albumen consistency over one week (Wakebe, 1998), two weeks (Pan et al., 2004) or a combination of organic minerals containing Sel-Plex® over 12 days (Xavier et al., 2004), an improvement in the quality of albumen through dietary Sel-Plex®, as measured by Haugh units, has been shown.
The beneficial effect of Sel-Plex® on albumen height represents protection of cellular membranes of the magnum, which are basically formed by cholesterol, phospholipids and peripheral and integral proteins (Vander et al., 1990). One of the roles of integral proteins is to serve as nutrient carriers to and from extracellular to intracellular fluid. Therefore, one may hypothesize that by protecting the membrane of secretory cells and tubular glands of the magnum, Se as a component of GSH-Px allows the secretory granules to discharge their contents more efficiently. The more protein gets into the magnum lumen, the more viscous the egg white becomes (Butts and Cunningham, 1972; Joseph et al., 2000). It should be noted that histological characteristics of the cells in the tubular glands indicate that they produce egg white proteins continuously throughout the ovulatory cycle and store the proteins in secretory granules (Etches, 1996).
As mentioned above, a reduction in albumen is observed when birds receive low-protein diets, suggesting that these diets are lower in essential amino acids (Butts and Cunningham, 1972; Joseph et al., 2000). Those proteins are produced in the rough endoplasmic reticulum and packaged by the Golgi apparatus. Then, proteins are transferred into secretory granules until the moment they are secreted into the magnum (Sandoz et al., 1971). Since rough endoplasmic reticulum and Golgi apparatus are cell organelles surrounded by membranes, membranes must be intact for proper function. This again indicates an important role of organic Se in improving Se status and consequently antioxidant status.
Conclusions
The beneficial effects of organic mineral supplements on broiler breeder performance are very consistent. Favorable responses have been observed at various ages of supplementation, however, it is advised to begin supplementation during the pre-lay diet and continue throughout the reproductive cycle.
References
Arnold, R.L., O.E. Olson and C.W. Carlson. 1974. Tissue selenium content and serum tocopherols as influenced by dietary type, selenium and vitamin E. Poult. Sci. 53:2185-2192.
Badawy, E.M., B.M. Edrise and A.M. Al-Wakeel. 1987. The relationship between quality, egg constituents and hatchability of the eggs of Hubbard broiler breeders:
a field study. Vet. Med. J. 35:105-115.
Barbato, G.F. 1999. Genetic relationships between selection for growth and reproductive effectiveness. Poult. Sci. 78:444-452.
Bekhtina, V.G. 1968. Morphological features of polyspermy fecundation in hens. In: Summaries from Pushkin Research Laboratory of Livestock Breeding. Leningrad Region, USSR. Cited by World’s Poult. Sci. J. 24:148.
Brake, J.T. 1996. Optimization of egg handling and storage. World Poult. Misset 12(9):33-39.
Brake, J., J.D. Garlich and E.D. Peebles. 1985. Effect of protein and energy intake by broiler breeders during the prebreeder transition period on subsequent reproductive performance. Poult. Sci. 64:2335-2340.
Brake, J., T. Walsh and S.V. Vick. 1993. Hatchability of broiler eggs as influenced by storage and internal quality. Zootech. Int. 16(1):30-41.
Brake, J., T.J. Walsh, C.E. Benton, J.N. Petitte, R. Meiherhof and G. Penalva. 1997. Egg handling and storage. Poult. Sci. 76:144-151.
Bramwell, R.K. and B. Howarth, Jr. 1992. Preferential attachment of cock spermatozoa to the perivitelline layer directly over the germinal disc of the hen’s ovum. Biol. Reprod. 47:1113-1117.
Bramwell, R.K., H.L. Marks and B. Howarth, Jr. 1995. Quantitative determination of spermatozoa penetration of the perivitelline layer of the hen’s ovum as assessed on oviposited eggs. Poult. Sci. 74:1875-1883.
Brillard, J.P. 2004. Natural mating in broiler breeders: present and future concerns. World’s Poult. Sci. J. 60:439-445.
Butts, J.N. and F.E. Cunningham. 1972. Effect of dietary protein on selected properties of the egg. Poult. Sci. 51:1726-1734.
Cantor, A.H. 1997.The role of selenium in poultry nutrition. In: Biotechnology in the Feed Industry, Proceedings of Alltech’s 13th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 155-164.
Cave, N.A.G. 1984. Effect of a high-protein diet fed prior to the onset of lay on performance of broiler breeder pullets. Poult. Sci. 63:1823-1827.
Edens, F.W. 2002. Practical applications for selenomethionine: broiler breeder reproduction. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 18th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 29-42.
Elibol, O. and J. Brake. 2004. Identification of critical periods for turning broiler hatching eggs during incubation. Br. Poult. Sci. 45:631-637.
Eslick, M.L. and G.R. McDaniel. 1992. Interelationships between fertility and hatchability of eggs from broiler breeder hens. J. Appl. Poult. Res. 1:156-159.
Etches, R.J. 1996. Reproduction in Poultry. 1st ed., CAB International, Wallingford, UK.
Fontana, E.A., W.D. Weaver, Jr. and H.P. van Krey. 1992. Intermittent periods of infertility identified in naturally mated broiler breeder hens. J. Appl. Poult. Res. 1:190-193.
Guo, Y.M., R. Yang, J. Yuan, T.L. Ward and T.M. Fakler. 2002. Effect of AvailaZn and ZnSO4 on laying hen performance and egg quality. Poult. Sci. 81(Suppl. 1):40.
Gyles, N.R. 1989. Poultry, people and progress. Poult. Sci. 68:1-8.
Hammerstedt, R.H. 1999. Symposium summary and challenges for the future. Poult. Sci. 78:459-466.
Havenstein, G.B., P.R. Ferket and M.A. Qureshi. 2003a.
Growth, livability and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82:1500-1508.
Havenstein, G.B., P.R. Ferket and M.A. Qureshi. 2003b. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82:1509-1518.
Hazary, R.C., H.J. Staines and G.J. Wishart. 2000. Assessing the mating efficiency and predicting fertility in broiler breeder flocks by quantifying spermatozoa which penetrate the inner perivitelline layer over the germinal disc. Br. Poult. Sci. 41:395-400.
Hudson, B.P., W.A. Dozier, J.L. Wilson, J.E. Sander and T.L. Ward. 2004a. Reproductive performance and immune status of caged broiler breeder hens provided diets supplemented with either inorganic or organic sources of zinc from hatching to 65 wk of age. J. Appl. Poult. Res. 13:349-359.
Hudson, B.P., B.D. Fairchild, J.L. Wilson, W.A. Dozier and J. Buhr. 2004b. Breeder age and zinc source in broiler breeder hen diets on progeny characteristics at hatching. Poult. Sci. 13:55-64.
Hurnik, G.L., B.S. Reinhart and J.F. Hurnik. 1978. Relationship between albumen quality and hatchability in fresh and stored eggs. Poult. Sci. 57:854-857.
Jacques, K.A. 2001. Selenium metabolism in animals: the relationship between dietary selenium form and physiological response. In: Science and Technology in the Feed Industry, Proceedings of Alltech’s 17th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), Nottingham University Press, UK, pp. 319-348.
Jensen, L.S. 1968. Selenium deficiency and impaired reproduction in Japanese quail. Proc. Soc. Exp. Biol. Med. 128:970-972.
Joseph, N.S., F.E. Robinson, D.R. Korver and R.A. Renema. 2000. Effect of dietary protein intake during the pullet-to-breeder transition period on early egg weight and production in broiler breeders. Poult. Sci. 79:1790-1796.
Kerr, C.L. 1998. Genetics of female-related fertility in chickens. PhD Dissertation. The Pennsylvania State University, University Park, PA.
Keshavarz, K. 1995. Further investigations on the effect of dietary manipulations of nutrients on early egg weight. Poult. Sci. 74:62-74.
Keshavarz, K. and S. Nakajima. 1995. The effect of dietary manipulations of energy, protein and fat during the growing and laying periods on early egg weight and egg components. Poult. Sci. 74:50-61.
Kirk, S.G., G.C. Emmans, R. McDonald and D. Arnot. 1980. Factors affecting the hatchability of eggs from broiler breeders. Br. Poult. Sci. 21:37-53.
Kuroki, M. and M. Mori. 1997. Binding of spermatozoa to the perivitelline layer in the presence of a protease inhibitor. Poult. Sci. 76:748-752.
Lanning, D., K. Ayres and S. Kenyon. 2000. Sel-Plex® in the breeder diet: reductions in chick mortality: summary of commercial studies in Britain 2000.
Alltech UK, Stampford, Lincs, UK. Lapão, C., T.L. Gama and M.C. Soares. 1999. Effects of broiler breeder age and length of egg storage on albumen characteristics and hatchability. Poult. Sci. 78:640-645.
Leeson, S. and J.D. Summers. 1997. Commercial Poultry Nutrition. 2nd ed., University Books, Guelph, Ontario, Canada.
Leeson, S. and J.D. Summers. 2000. Broiler Breeder Production. 1st ed., University Books, Guelph, Ontario, Canada.
Leeson, S. and J.D. Summers. 2001. Scott’s Nutrition of the Chicken. 4th ed., University Books, Guelph, Ontario, Canada.
Lopez, G. and S. Leeson. 1994. Nutrition and broiler breeder performance: a review with emphasis on response to diet protein. Appl. Poult. Res. 3:303-311.
Maas, J. 1999. Trace mineral requirements of cattle and poultry. In: Proc. Calif. Anim. Nutr. Conf., Fresno, May 5-6, pp. 39-48.
Mahan, D.C. and N.A. Parret. 1996. Evaluating the efficiency of selenium-enriched yeast and sodium selenite on tissue selenium retention and serum glutathione peroxidase activity in grower and finisher swine. J. Anim. Sci. 74:2967-2974.
McDaniel, G.R., J. Brake and M.K. Eckman. 1981. Factors affecting broiler breeder performance. 1. Relationship of daily feed intake level to reproductive performance of pullets. Poult. Sci. 60:307-312.
McDowell, L.R. 2003. Minerals in Animal and Human Nutrition. 2nd ed., Elsevier Science B.V., Amsterdam, pp 644.
Meuer, H.J. and R. Baumann. 1988. Oxygen pressure in intra and extraembryonic blood vessels of early chick embryos. Respir. Physiol. 71:331-342.
Noble, R.C. and M. Cocchi. 1990. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 29:107-
Pan, E.A., F. Rutz, N.J.L. Dionello, M.A. Anciuti and R.R. da Silva. 2004. Performance of brown egg layers fed diets containing organic selenium (Sel-Plex®). In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of the 20th Annual Symposium (Suppl. 1: Abstracts of posters presented). Lexington, Ky, May 24-26, p. 18.
Pappas, A.C., R.M. McDevitt, P.F. Surai, T. Acamovic and N.H.C. Sparks. 2004. The effects of selenium and PUFA supplementation in the diet of young broiler breeders on the incorporation of selenium in the egg and in the tissues of day old broiler chick. Br. Poult. Sci. S26-27(Suppl.).
Paton, N.D., A.H. Cantor, A.J. Pescatore, M.J. Ford, C.A. Smith. 2002. The effect of dietary selenium source and level on the uptake of selenium by developing chick embryos. Poult. Sci. 81:1548-1554.
Pedrieri, G. and E. Cinti. 2003. New metal chelates for animal nutrition. www.feedinfo.com (dated 20 June 2003; accessed 19 September 2003), 4 pages.
Pinchasov, Y. 1991. Relation between the weight of hatching eggs and subsequent early performance of broiler chicks. Br. Poult. Sci. 32:109-115.
Rahn, H. and C.V. Paganelli. 1991. Energy budget and gas exchange of avian eggs. In: Avian Incubation (S.G. Tullet, ed). Buttersworth and Heinemann, London, UK, pp. 175-193.
Rebel, J.M.J., T.P. van Dam, B. Zekarias, F.R. M. Balk, J. Post, A. Flores Minambres and A.A.H. M. ter Huurne. 2004. Vitamin and trace mineral content in feed of breeders and their progeny: effects of growth, feed conversion and severity of malabsorption syndrome of broilers. Br. Poult. Sci. 45:201-209.
Reddy, R.P. and M. Sadjadi. 1990. Selection for growth and semen traits in the poultry industry: what can we expect in the future. In: Control of Fertility in Domestic Species (J.P. Brillard, ed). INRA editions, Vol. 54, pp. 47-59.
Renema, R.A. 2004. Reproductive responses to Sel- Plex® organic selenium in male and female broiler breeders: impact on production traits and hatchability. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 20th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp 81-91.
Renema, R.A. and F.E. Robinson. 2004. Defining normal: comparison of feed restriction and full feeding of female broiler breeders. World’s Poult. Sci. 60:508-522.
Romanoff, A.L. 1960. The Avian Embryo: Structure and Functional Development. MacMillan, New York, NY.
Rutz, F., E.A. Pan, G.B. Xavier and M.A. Anciuti. 2003. Meeting selenium demands of modern poultry:
responses to Sel-Plex® organic selenium in broiler and breeder diets. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 19th Annual Symposium (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK, pp. 165-185.
Sandoz, D., E. Ulrich and E. Brard. 1971. Etude des ultraestructures du magnum des oiseaux. Evolution au cours du cycle de ponte chez la poule Gallus domesticus. J. de Microscopie 11:371-400.
Shanawany, M.M. 1987. Hatching weight in relation to egg weight in domestic birds. World’s Poult. Sci. J. 43:107-115.
Surai, P.F. 2000. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 41:235-243.
Surai, P.F. 2002. Natural Antioxidants in Avian Nutrition and Reproduction. 1st ed., Nottingham University Press, UK.
Surai, P.F. and N.H.C. Sparks. 2000. Effect of the selenium content of the maternal diet on the antioxidant system of the yolk. Department of Biochemistry and Nutrition, Scottish Agricultural College Auchincruive, Ayr, KA65HN, Scotland, UK, Br. Soc. Anim. Sci.
Suso, F.A. and H.M. Edwards, Jr. 1972. Binding of EDTA, histidine and acetylsalicylic acid to zincprotein complex in intestinal content, intestinal mucosa and blood plasma. Nature 236:230-232.
Tona, K., O. Onagbesan, B. de Ketelaere, E. Decuypere and V. Bruggeman. 2004. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight and chick posthatch growth to 42 days. J. Appl. Poult. Res. 13:10-18.
Vander, A.J., J.H. Sherman and D.S. Luciano. 1990. Human Physiology. 5th ed., McGraw-Hill Publishing Co., New York, NY.
Vieira, S.L. and E.T. Moran. 1998. Eggs and chicks from broiler breeders of extremely different age. J. Appl. Poult. Res. 7:372-376.
Wakebe, M. 1998. Organic selenium and egg freshness. Patent No. 10-23864. Feed for meat chicken and feed for laying hens. Japanese Patent Office, Application Heisei 8-179629. Published Jan 27.
Wilson, H.R. 1997. Effects of maternal nutrition on hatchability. Poult. Sci. 76:134-143.
Wishart, G.J. and H.J. Staines. 1999. Measuring sperm:egg interaction to assess breeding efficiency in chickens in chickens and turkeys. Poult. Sci. 87:412- 466.
Xavier, G.B., F. Rutz, N.J.L. Dionello, A.D. Duarte, F.M. Goncalves and N.H.F. Zauk and C. L. G. Ribeiro. 2004. Performance of layers fed diets containing organic selenium, zinc and manganese, during a second cycle of production. In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of the 20th Annual Symposium (Suppl. 1: Abstracts of posters presented). Lexington, Ky, May 24-26, p. 19.
Authors: FERNANDO RUTZ, MARCOS ANTONIO ANCIUTI, JOSE LUIZ RECH and EDUARDO GONÇALVES XAVIER
Departamento de Zootecnia, Universidade Federal de Pelotas, Brazil







.jpg&w=3840&q=75)