Excreta biomarkers in response to different gut barrier dysfunction models and probiotic supplementation in broiler chickens
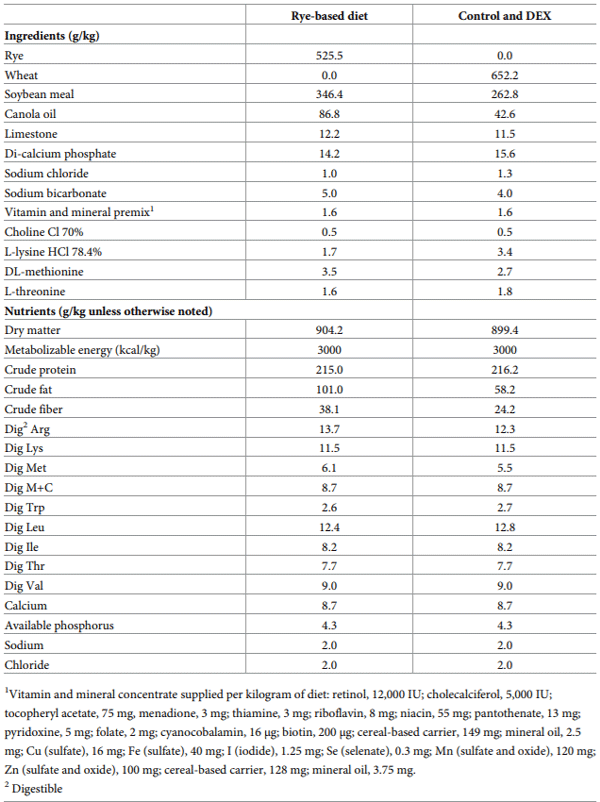
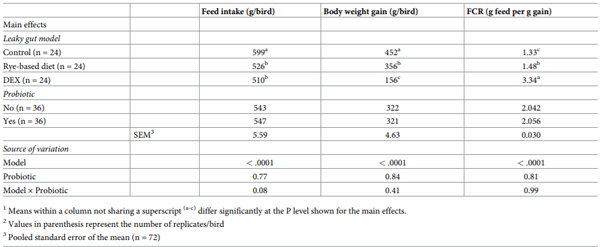
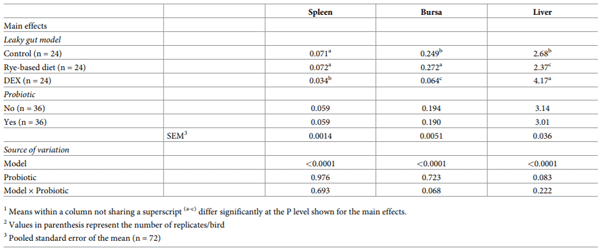
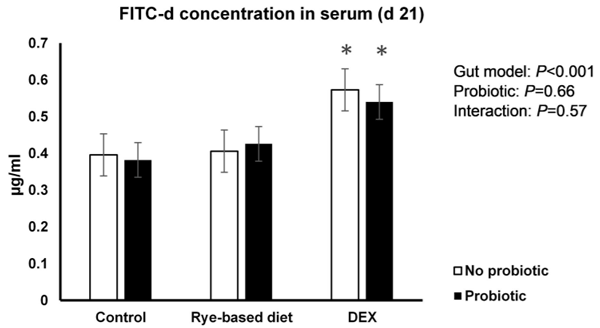
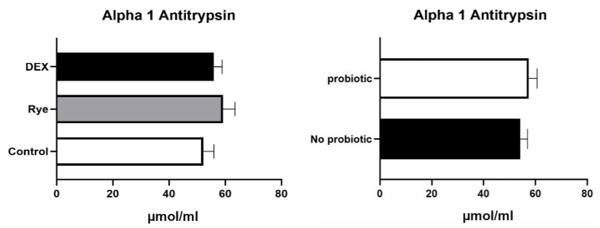
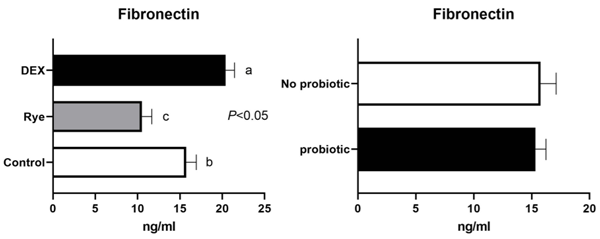
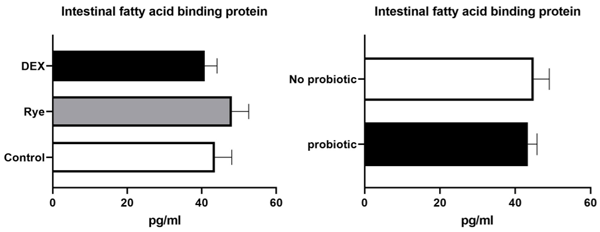
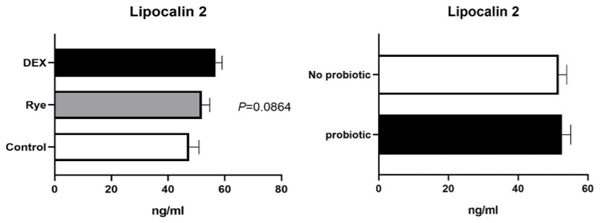
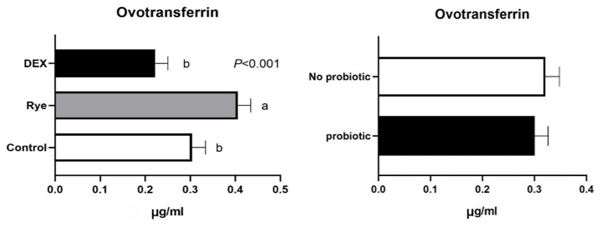
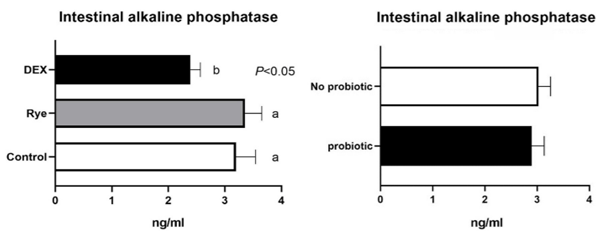
Increased intestinal permeability (IP) and inflammation are both linked with functionality of the intestinal barrier and in particular enterocytes. Currently, almost all assessment methods of the intestinal barrier function are invasive. The present study aimed to quantify selected proteins as novel biomarkers in excreta of broiler chickens to facilitate non-invasive assessment of gut barrier function using enzyme-linked immunosorbent assays (ELISA). It was further hypothesised that probiotics as feed additives may counteract gut barrier dysfunction. A 3 × 2 factorial arrangement of treatments was used with the main factors being gut barrier dysfunction models (control, rye-based diet, and dexamethasone–DEX) with and without probiotic supplementation (a three-strain Bacillus) using 72 male Ross 308 day-old chick-ens. Each of the 6 experimental treatments was replicated 12 times. On d 21 of age, fluorescein isothiocyanate dextran (FITC-d) uptake into serum was examined to test IP. Fresh excreta samples were collected on d 20. The biomarkers included alpha-1 antitrypsin (A1AT), intestinal fatty acid binding protein (I-FABP), lipocalin-2 (LCN2), fibronectin (FN), intestinal alkaline phosphatase (IAP), ovotransferrin (OVT) and superoxide dismutase [Cu-Zn] (SOD1). Only DEX increased (P<0.001) FITC-d passage to the blood on d 21 of age, indicating a greater IP. The excreta concentrations of A1AT, I-FABP and SOD1 were unaltered by the experimental treatments. DEX increased (P<0.05) FN concentration in excreta compared with control birds. Conversely, inclusion of rye in the diet reduced (P<0.05) FN but increased (P<0.001) OVT in excreta. Independently, DEX decreased IAP (P<0.05) in excreta compared with control and rye-fed birds. The excreta concentration of LCN2 tended (P = 0.086) to increase in birds injected by DEX. There was no demonstrable effect of pro-biotic addition on any of the studied parameters. Among the tested biomarkers, FN, IAP, and LCN2 revealed promise as biomarkers of intestinal barrier function quantified by ELISA kits.
![Fig 8. Concentration of superoxide dismutase [Cu-Zn] in excreta samples (n = 36) for the main effects of gut barrier dysfunction models (P> 0.05) and probiotic supplementation (P> 0.05). Error bars represent standard error of the mean (SEM).](/_next/image/?url=https%3A%2F%2Fimages.engormix.com%2FE_articles%2F48513_630.gif&w=1200&q=75)
- Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017; 11:821–34. https://doi.org/10.1080/17474124.2017.1343143 PMID: 28650209
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junc-tion permeability by intestinal bacteria and dietary components. J Nutr. 2011; 141:769–76. https://doi.org/10.3945/jn.110.135657 PMID: 21430248
- Gilani S, Howarth GS, Kitessa SM, Forder RE, Tran CD, Hughes RJ. New biomarkers for intestinal per-meability induced by lipopolysaccharide in chickens. Anim Prod Sci. 2016; 56:1984–97.
- De Meyer F, Eeckhaut V, Ducatelle R, Dhaenens M, Daled S, Dedeurwaerder A, et al. Host intestinal biomarker identification in a gut leakage model in broilers. Vet Res. 2019; 50:46. https://doi.org/10.1186/s13567-019-0663-x PMID: 31215487
- Ducatelle R, Goossens E, De Meyer F, Eeckhaut V, Antonissen G, Haesebrouck F, et al. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet Res. 2018; 49:43. https://doi.org/10.1186/s13567-018-0538-6 PMID: 29739469
- Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, et al. Fecal markers of intestinal inflamma-tion and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013; 88:390–6. https://doi.org/10.4269/ajtmh.2012.12-0549 PMID: 23185075
- Celi P, Verlhac V, Calvo EP, Schmeisser J, Kluenter A-M. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim Feed Sci Technol. 2019; 250:9–31.
- Sikora M, Stec A, Chrabaszcz M, Waskiel-Burnat A, Zaremba M, Olszewska M, et al. Intestinal fatty acid binding protein, a biomarker of intestinal barrier, is associated with severity of psoriasis. J Clin Med. 2019; 8:1021.
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432:917. https://doi.org/10.1038/nature03104 PMID: 15531878
- Goossens E, Debyser G, Callens C, De Gussem M, Dedeurwaerder A, Devreese B, et al. Elevated fae-cal ovotransferrin concentrations are indicative for intestinal barrier failure in broiler chickens. Vet Res. 2018; 49:51. https://doi.org/10.1186/s13567-018-0548-4 PMID: 29925427
- Baxter MF, Latorre JD, Dridi S, Merino-Guzman R, Hernandez-Velasco X, Hargis BM, et al. Identifica-tion of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model. Front Vet Sci. 2019; 6:144. https://doi.org/10.3389/fvets.2019.00144 PMID: 31143767
- Thomas DW, Henton DH. The use of fecal alkaline phosphatase as an indicator of intestinal damage. Digestion. 1985; 31:82–8. https://doi.org/10.1159/000199184 PMID: 2581839
- Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, et al. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Micro-biol. 2018; 9:1953. https://doi.org/10.3389/fmicb.2018.01953 PMID: 30197632
- Gadde U, Oh S, Lee Y, Davis E, Zimmerman N, Rehberger T, et al. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob Proteins. 2017; 9:397–405. https://doi.org/10.1007/s12602-017-9275-9 PMID: 28421423
- Zuber T, Miedaner T, Rosenfelder P, Rodehutscord M. Amino acid digestibility of different rye geno-types in caecectomised laying hens. Arch Anim Nutr. 2016; 70:470–87. https://doi.org/10.1080/1745039X.2016.1226035 PMID: 27618757
- Latorre J, Hernandez-Velasco X, Bielke L, Vicente J, Wolfenden R, Menconi A, et al. Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composi-tion and bone mineralisation in broiler chickens fed on a rye-based diet. Br Poult Sci. 2015; 56:723–32. https://doi.org/10.1080/00071668.2015.1101053 PMID: 26539833
- Barekatain R, Nattrass G, Tilbrook AJ, Chousalkar K, Gilani S. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without syn-thetic glucocorticoid. Poult Sci. 2019; 98:3662–75. https://doi.org/10.3382/ps/pey563 PMID: 30597073
- Wideman R Jr, Pevzner I. Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poult Sci 2012; 91:2464–74. https://doi.org/10.3382/ps.2012-02386 PMID: 22991529
- Gilani S, Howarth GS, Tran CD, Barekatain R, Kitessa SM, Forder REA, et al. Reduced fasting periods increase intestinal permeability in chickens. J Anim Physiol Anim Nutr. 2018; 102:e486–e492.
- Duff AF, Baxter MF, Graham BD, Hargis BM, Bielke LR. Mode of action of dietary dexamethasone may not be dependent upon microbial mechanisms in broilers. Microorganisms. 2019; 7:346.
- Unsal Hm, Balkaya M. Glucocorticoids and the intestinal environment. Glucocorticoids-New Recogni-tion of Our Familiar Friend: InTech Open Access Publisher; 2012.
- Tellez G, Latorre JD, Kuttappan VA, Kogut MH, Wolfenden A, Hernandez-Velasco X, et al. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front Genet. 2014; 5:339. https://doi.org/10.3389/fgene.2014.00339 PMID: 25309584
- Kuttappan VA, Vicuña EA, Latorre JD, Wolfenden AD, Te´llez GI, Hargis BM, et al. Evaluation of gastro-intestinal leakage in multiple enteric inflammation models in chickens. Front Vet Sci. 2015; 2:66. https://doi.org/10.3389/fvets.2015.00066 PMID: 26697435
- Barekatain R, Chrystal PV, Howarth GS, McLaughlan CJ, Gilani S, Nattrass GS. Performance, intesti-nal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poult Sci. 2019; 98:6761–71. https://doi.org/10.3382/ps/pez393 PMID: 31328774
- Gharib Naseri K, Swick R, Choct M, Morgan N, Wu S. Impact of grain type on performance and gut microbiota composition in broilers under necrotic enteritis challenge. Proceeding of the 29th Australian Poultry Science Symposium. 2018; 29:176–8.
- Hernandez-Patlan D, Solis-Cruz B, Pontin KP, Hernandez X, Merino-Guzman R, Adhikari B, et al. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions and ileal microbiota in broiler chickens using a laboratory challenge model. Front Vet Sci. 2019; 6:108. https://doi.org/10.3389/fvets.2019.00108 PMID: 31106209
- Song J, Xiao K, Ke Y, Jiao L, Hu C, Diao Q, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult Sci. 2014; 93:581–8. https://doi.org/10.3382/ps.2013-03455 PMID: 24604851
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008; 7:489–503. https://doi.org/10.1038/nrd2589 PMID: 18511927
- Muga A, Cistola DP, Mantsch HH. A comparative study of the conformational properties of Escherichia coli-derived rat intestinal and liver fatty acid binding proteins. Biochimica et Biophysica Acta (BBA)— Protein Structure and Molecular Enzymology. 1993; 1162:291–6.
- Adriaanse M, Tack G, Passos VL, Damoiseaux J, Schreurs M, Wijck K, et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibod-ies. Aliment Pharmacol Ther. 2013; 37:482–90. https://doi.org/10.1111/apt.12194 PMID: 23289539
- Chen J, Tellez G, Richards JD, Escobar J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front Vet Sci. 2015; 2:14. https://doi.org/10.3389/fvets.2015.00014 PMID: 26664943
- Reisinger KW, Van der Zee DC, Brouwers HA, Kramer BW, van Heurn LE, Buurman WA, et al. Nonin-vasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid–bind-ing protein in necrotizing enterocolitis. J Pediatr Surg. 2012; 47:1640–5. https://doi.org/10.1016/j.jpedsurg.2012.02.027 PMID: 22974599
- Ni L, Chen Q, Zhu K, Shi J, Shen J, Gong J, et al. The influence of extracorporeal membrane oxygen-ation therapy on intestinal mucosal barrier in a porcine model for post-traumatic acute respiratory dis-tress syndrome. J Cardiothorac Surg. 2015; 10:20. https://doi.org/10.1186/s13019-015-0211-3 PMID: 25884385
- Zhu W, Li L, Deng M, Wang B, Li M, Ding G, et al. Oxidation-resistant and thermostable forms of alpha-1 antitrypsin from Escherichia coli inclusion bodies. FEBS Open Bio. 2018; 8:1711–21. https://doi.org/10.1002/2211-5463.12515 PMID: 30338221
- Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bu¨ rmann J, Faßbender K, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord. 2018; 50:104–7. https://doi.org/10.1016/j.parkreldis.2018.02.022 PMID: 29454662
- Wang L, Llorente C, Hartmann P, Yang A-M, Chen P, Schnabl B. Methods to determine intestinal per-meability and bacterial translocation during liver disease. Parkinsonism Relat Disord. 2018; 50:104–7. https://doi.org/10.1016/j.parkreldis.2018.02.022
- Gilani S, Howarth GS, Kitessa SM, Tran CD, Forder REA, Hughes R. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. J Anim Physiol Anim Nutr. 2017; 101:e237–e245.
- Abella V, Scotece M, Conde J, Go´mez R, Lois A, Pino J, et al. The potential of lipocalin-2/NGAL as bio-marker for inflammatory and metabolic diseases. Biomarkers. 2015; 20:565–71. https://doi.org/10.3109/1354750X.2015.1123354 PMID: 26671823
- Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PloS One. 2012; 7: e44328. https://doi.org/10.1371/journal.pone.0044328 PMID: 22957064
- Agus A, Denizot J, The´venot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016; 6:19032. https://doi.org/10.1038/srep19032 PMID: 26742586
- Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, et al. Homeostasis of the gut bar-rier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2016; 312:G171–G193. https://doi.org/10.1152/ajpgi.00048.2015 PMID: 27908847
- Gare´naux A, Houle S, Folch B, Dallaire G, Truesdell M, Le´pine F, et al. Avian lipocalin expression in chickens following Escherichia coli infection and inhibition of avian pathogenic Escherichia coli growth by Ex-FABP. Vet Immunol Immunopathol. 2013; 152:156–67. https://doi.org/10.1016/j.vetimm.2012.09.018 PMID: 23102565
- Owen HC, Roberts SJ, Ahmed SF, Farquharson C. Dexamethasone-induced expression of the gluco-corticoid response gene lipocalin 2 in chondrocytes. Am J Physiol Endocrinol Metab. 2008; 294:E1023– E1034. https://doi.org/10.1152/ajpendo.00586.2007 PMID: 18381927
- Rath N, Anthony N, Kannan L, Huff W, Huff G, Chapman H, et al. Serum ovotransferrin as a biomarker of inflammatory diseases in chickens. Poult Sci. 2009; 88:2069–2074. https://doi.org/10.3382/ps.2009-00076 PMID: 19762858
- Feng Y-L, Tang X-L. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem Biol Interact. 2014; 207:26–31. https://doi.org/10.1016/j.cbi.2013.11.004 PMID: 24239970
- Dhanani KCH, Samson WJ, Edkins AL. Fibronectin is a stress responsive gene regulated by HSF1 in response to geldanamycin. Sci Rep. 2017; 7:17617. https://doi.org/10.1038/s41598-017-18061-y PMID: 29247221
- Kolachala VL, Bajaj R, Wang L, Yan Y, Ritzenthaler JD, Gewirtz AT, et al. Epithelial-derived fibronectin expression, signaling, and function in intestinal inflammation. J Biol Chem. 2007; 282:32965–73. https://doi.org/10.1074/jbc.M704388200 PMID: 17855340
- McKeown-Longo PJ, Etzler CA. Induction of fibronectin matrix assembly in human fibrosarcoma cells by dexamethasone. J Cell Biol. 1987; 104:601–10. https://doi.org/10.1083/jcb.104.3.601 PMID: 2950120
- Kono I, Kitao H, Matsuda M, Haga S, Fukushima H, Kashiwagi H. Weight reduction in athletes may adversely affect the phagocytic function of monocytes. Phys Sportsmed. 1988; 16:56–65. https://doi. org/10.1080/00913847.1988.11709552 PMID: 27403825
- Sa´nchez B, Arias S, Chaignepain S, Denayrolles M, Schmitter J-M, Bressollier P, et al. Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology. 2009; 155:1708–16. https://doi.org/10.1099/mic.0.025288-0 PMID: 19372165
- Styriak I, Nemcova R, Chang YH, Ljungh Å. Binding of extracellular matrix molecules by probiotic bacte-ria. Lett Appl Microbiol. 2003; 37:329–33. https://doi.org/10.1046/j.1472-765x.2003.01402.x PMID: 12969498
- Lallès J-P. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014; 72:82–94. https://doi.org/10.1111/nure.12082 PMID: 24506153
- Al-Zghoul MB, Alliftawi AReS, Saleh KMM, Jaradat ZWJPs. Expression of digestive enzyme and intesti-nal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult Sci. 2019; 98:4113–22. https://doi.org/10.3382/ps/pez249 PMID: 31065718
- Lallès J-P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr Rev. 2019; 77:710–24. https://doi.org/10.1093/nutrit/nuz015 PMID: 31086953
- Lallès J-P. Intestinal alkaline phosphatase in stool: a novel biomarker for metabolic diseases. EBioMedicine. 2015; 2:1866. https://doi.org/10.1016/j.ebiom.2015.12.001 PMID: 26844263
- McCormick BJ, Lee GO, Seidman JC, Haque R, Mondal D, Quetz J, et al. Dynamics and trends in fecal biomarkers of gut function in children from 1–24 months in the MAL-ED study. Am J Trop Med Hyg. 2017; 96:465–72. https://doi.org/10.4269/ajtmh.16-0496 PMID: 27994110










00167-168.jpg&w=3840&q=75)