I. INTRODUCTION
Oregano essential oil contains many compounds, of which carvacrol, thymol and their precursors are the major components, accounting for approximately 80% of the contents. Carvacrol and thymol have been shown to actively disrupt the cell membranes of bacteria leading to cell death, promoting the use of oregano as a phytobiotic (Rao et al., 2010). Phytoadditives such as oregano are becoming more popular as organic and natural alternatives to antibiotics. Many commercial poultry feed supplements are based on oregano antimicrobial products.
Although there is evidence of the presence of bacteria in ovo, the bulk of the microbiota colonisation of the chicken gastrointestinal tract starts from hatch, resulting in a highly populated gut within three days (Lu et al., 2003; Apajalahti et al., 2016). Commercial chickens have a highly variable microbiota (Stanley et al., 2012) that could be easily influenced by various feed additives and feed ingredients during first days of colonisation.
Numerous studies have investigated the effects of oregano on human and animal pathogens. Akdemir Evrendilek (2015) reported that oregano could inhibit growth of Listeria innocua, coagulase-negative staphylococci, Staphylococcus aureus, Bacillus subtilis, Yersinia enterocolitica, Salmonella Enteritidis, Salmonella Typhimurium, Proteusmirabilis, Escherichiacoli O157:H7, and Klebsiellaoxytoca. Silva et al., (2013) tested essential oils of known antibacterial herbs including thyme, oregano, rosemary, verbena, basil, peppermint, pennyroyal and mint for their activity against the food spoilage bacteria. Oregano showed the highest antimicrobial activity against the food spoilage bacteria Listeria monocytogenes, Clostridium perfringens, Bacillus cereus, S. aureus, Enterococcus faecium, Enterococcus faecalis, Staphylococcus epidermidis, Salmonella enterica, E. coli and Pseudomonasaeruginosa. Others suggested that oregano’s main active ingredient, carvacrol, can control biofilm formation by disrupting bacterial quorum sensing (Burt et al., 2014).
With the established ability of oregano to control some of the major poultry pathogens, we hypothesised that a high concentration of oregano in the feed given from day 1 would remodel gut development and prevent, or reduce, the colonisation of some major poultry pathogens such as Salmonella and Campylobacter species.
II. METHOD
Oregano and feed preparation: Dried oregano leaves (Saucy Spice Company, North Boambee Valley, NSW, Australia) were blended (100 g, 1.5 min/max, 1500W, Nutri Ninja Auto iQ Duo, SharkNinja, USA) and processed in a Planetary Ball Mill Machine (speed no. 5, 2 hrs, 40 g per each run, Changsha Yonglekang Equipment, China). The oregano was then sized using an electric sieve machine (Changsha Yonglekang Equipment, China) and the particle size of the powder was determined by laser diffraction (Mastersizer 2000, Malvern, ATA scientific, Australia); the average size was 10 μm. Antimicrobial and coccidiostat free chicken starter diet crumble (Red Hen, Laucke Mills, Australia) was formulated to meet or exceed the National Research Council guidelines for broiler chickens (NRC, 1994). The 2% of oregano (0.02 kg/kg w/w), was evenly distributed through the feed using an electric mixer (125 L mixer, Ozito, China).
Birds and management: The study was approved by the Animal Ethics Committee at Central Queensland University under the approval number 0000020312 and the data on microbiota structure of different intestinal sections (ileum, jejunum and caecum) at slaughter day at 6 week old with pathogen reduction profiles in these sections, histology and SCFA profiles have been published separately (Bauer et al, 2019). Day-old broiler chicks (Ross Broiler 308, Bond Enterprises, Toowoomba) were delivered to the experimental facility and randomly distributed into two treatments, control (n = 12) and 2% w/w oregano (n=12). All birds were fed ad libitum and had unrestricted access to drinking water. The purpose of this experiment was not to evaluate bird performance, but the development of the microbial communities. Birds were individually tagged using coloured leg rings. Feed consumption and individual bird weights were measured each week, for six weeks. Freshly voided faecal samples (not swabs) were taken weekly from each birds separately.
DNA extraction and 16S rRNA sequencing: DNA was extracted from faecal samples for 16S rRNA gene sequencing. The detailed protocol has been previously described (Gangadoo et al., 2019). The primers contained barcodes, spacers, and Illumina sequencing linkers that have been previously described (Fadrosh et al., 2014). The 16S rRNA gene sequencing library preparation and amplification followed the Illumina recommended protocol (Illumina Inc., San Diego, CA, USA). Sequencing was conducted on the Illumina MiSeq platform using 2x300 bp paired-end sequencing. The sequence data generated from each sample were initially analysed using Quantitative Insights Into Microbial Ecology (QIIME v.1.9.1) as previously described (Gangadoo et al., 2019). All OTUs with less than 0.01% abundance were removed. A Hellinger transformed OTU table was used in statistical investigation. Significantly differentially abundant taxa were identified using ANOVA, and linear regression using Pearson’s correlation was also investigated. Calypso was used for further data exploration and visualization (Zakrzewski et al., 2017). The sequence data is publicly available on the MG-RAST database under project mgp89580 and library accession number mgl745316.
III. RESULTS
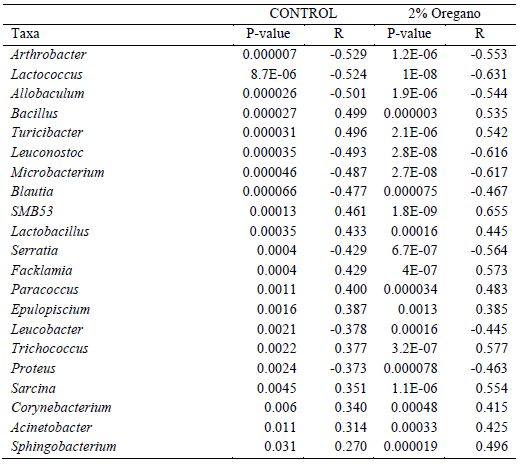
Oregano and the timeline of microbiota development: The overall structure of the faecal microbiota did not differ significantly between control and oregano supplemented communities (Adonis on weighted UniFrac matrix) at week 1 (P = 0.234), week 2 (P = 0.689), week 3 (P = 0.648), week 4 (P = 0.405), or week 5 (P = 0.176); however, they differentiated at week 6 (P = 0.036). The number of differentially abundant taxa at the genus level was not very high at any of the weekly data, and different genera were affected every week, for example, Lactobacillus was reduced in the oregano supplemented group 4.9 fold during week 3 only, and not significantly affected after that. There were also a range of pathogen-rich genera equally inconsistently reduced in oregano at multiple different sampling points, for example, Streptococcus was reduced only in week 1, Clostridium only in week 3 or Staphylococcus only in week 4. However, the genus correlation with the age of birds, across all 6 weeks, was not different in oregano supplemented birds when compared to the un-supplemented control. All significantly correlated genera (Pearson) changed with age in the same direction in both control and oregano supplemented birds (Table 1), significantly increasing with the birds' age in both control and oregano, or reducing in both groups. Oregano supplementation was not able to change the correlation direction or significance even in those taxa that were significantly reduced in abundance in the oregano supplemented birds at particular sampling times.
Table 1 - Pearson-based correlations of genera with the birds’ age, performed separately for control and oregano birds. Correlations were not reversed or removed by supplemented oregano but instead remained in the same direction. Every genus that increased over time in the control group was also significantly increased over time in the oregano group and vice versa. Significant positive correlations are shown in italic font and negative in bold.
IV. DISCUSSION
Oregano based products are among the most frequently used antibiotic alternatives in poultry farming with positive anecdotal feedback in terms of farmer perceived pathogen control. There is an abundance of in vitro studies showing that oregano can inhibit a range of common livestock and poultry pathogens, indicative that oregano would be expected to influence the development of gut microbiota. Although the microbial communities in the excreta became significantly different by week 6, we found that oregano did not prevent the gradual increase/decline of any microbial taxa or reverse its temporal development. At best, oregano in the formulation, at the level tested here, influenced the extent of such gradual changes in microbiota which in turn resulted in slightly differential microbial communities by the end of 6 weeks of continual oregano supplementation. An interesting outcome in this study was the variability of the differential microbiota from week to week. This raises several questions regarding the reliability of significantly differential taxa when there are no significant overall community differences, as well as the importance of timing of the sampling and diet influence.
A critical criterion for a phytochemical to be considered as a viable replacement for antibiotics is a minimal disturbance of the intestinal community and, ideally, high inhibitory preference towards poultry pathogens. In the current study, oregano did not disturb the microbial communities over time, even at the high dose of 2% supplementation. In the separate publication (Bauer et al., 2019) we published consistent reduction of some pathogens across gut sections (ileum, jejunum and caecum). Weekly faecal data indicate that oregano supplementation inhibited some pathogens, however not reproducibly over time, and did not exhibit any major influence on the temporal microbiota development trends. The finding that using faecal samples, different genera were affected every week may be result of well-established faecal microbiota sample variability (Stanley et al., 2015) that is a result of periodic emptying of different gut sections in chicken, temporal microbiota fluctuation or simply methodology noise. Either way, our results indicate that intestinal samples, such as caecum, are more appropriate to detect subtle differences in microbiota in chicken than are faecal samples.
Presented at the 31th Annual Australian Poultry Science Symposium 2020. For information on the next edition, click here.