Effects of Graded Levels of Alphamune G on Performance of Growing Pullets to Laying Hens
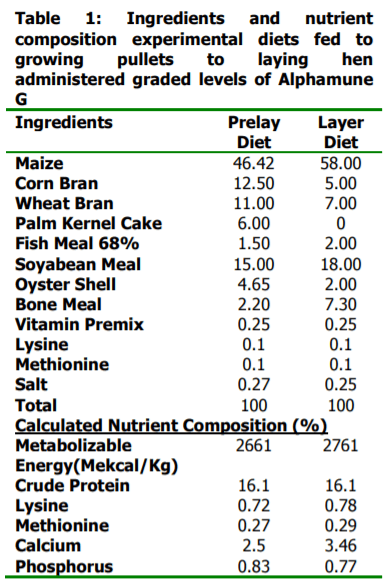
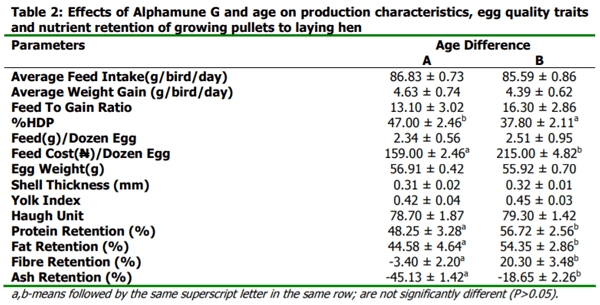
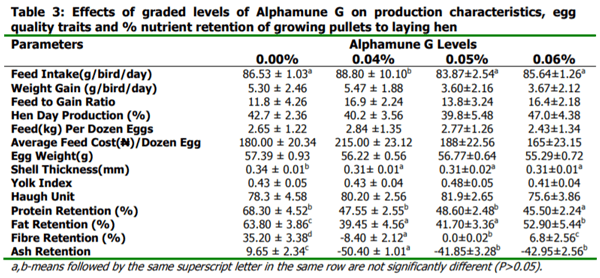
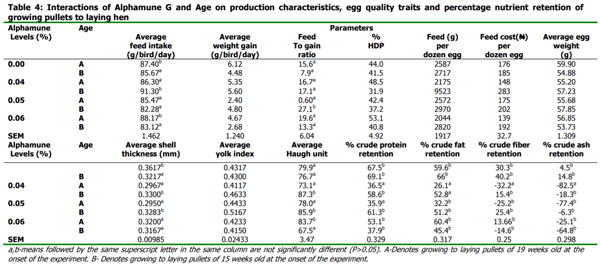
The study was conducted to investigate the effects of Alphamune G fed to growing pullets on their performance during laying. A total of one hundred and forty four (144) commercial black Harco pullet chickens made up of seventy-two (72) each of age 19 weeks old (group A) as well as age 15 weeks old (group B) were fed four graded levels of Alphamune G (0.00, 0.04, 0.05 and 0.06 % inclusion level). A factorial arrangement of four levels of dietary Alphamune G and two age groups were the treatments in a Complete Randomized Design. The study was conducted for 17 weeks, when the birds of each of group A and B attained age 36 weeks and 32 weeks, respectively. Laying pullets fed the control diet 0.00% had the highest value of egg weight, shell thickness and feed intake. Highest values for feed to gain ratio, feed per dozen eggs and feed cost per dozen eggs were observed in laying pullets fed 0.04% inclusion level of Alphamune G. The haugh unit, yolk index and weight gain were observed to have the highest value in layers fed 0.05%. Highest values was observed for % hen day production for laying hens fed 0.06% inclusion level of Alphamune G. In conclusion, among laying hens fed dietary levels of Alphamune G, birds fed inclusion levels of 0.06% performed the best in terms of production characteristics, egg quality and economic value compared to 0.04% and 0.05%.
Keywords: Alphamune G, Growing pullets, Laying hen, Production characteristics, Egg quality, Economic value
AGHAEI, A., TABATABAEI, S., CHAJI, M. and NAZARI, M. (2010). Effects of dried whey (prebiotics) and probiotics in laying hen’s performance and intestinal flora. Journal of Animal and Veterinary Advances, 9(15): 1996 – 2000.
ALPHARMA ANIMAL HEALTH (2004). Alphamune G for Poultry. http://www.thepoultrysite.com/articles/ 722/alpharmune-g-data-sheet(retrieved. Accessed April 04, 2009. AOAC. (1990). Official Methods of Analysis. 15th Edition, Association of Official Analytical Chemist Washington DC.
APPLEGATE, T., LADWIG, J. E., WEISSERT, L. and LILBURN, M. S. (1999). Effect of hen age on intestinal development and glucose tolerance of the Pekin duckling. Poultry Science, 78: 1485 – 1492.
ATTEH, J. O. (2004). Theory and Practice of Poultry. Adlek Publisher, Sabo-Oke, Ilorin, Nigeria.
BALEVI, T., UÇAN, U. S., COSKUN, B., KURTOGLU, V. and CETINGÜL, I. S. (2001). Effect of dietary probiotic on performance and humoral immune response in layer hens. British Poultry Science, 42(4): 456 – 461.
BOLU, S. A., OJO, V., OYELEKE, B. A., AJIBOYE, A. O., BAA SAMBO A. and OLUYEMI, O. (2009a). Response of broiler chicks to graded levels of Alphamune G supplementation. International Journal of Poultry Science, 8(1): 32 – 34.
BOLU, S. A., OJO, V., OLUYEMI, O., BABAWALE O. I. and AWODELE, O. A. (2009b). Effects of graded levels Alphamune G on performance, blood chemistry and histology of cockerel chicks. International Journal of Poultry Science, 8(4): 397 – 400.
CHEN, Y. C. and CHEN, T. C. (2004). Mineral utilization in layers as influenced by dietary oligofructose and insulin. International Journal of Poultry Science, 3(7): 442 – 445.
CHEN, Y. C., NAKTHONG, C. and CHEN, T. C. (2005a). Effects of chicory fructans on egg cholesterol in commercial laying hen. International Journal of Poultry Science, 4: 109 – 114.
CHEN, Y. C., NAKTHONG, C. and CHEN, T. C. (2005b). Improvement of laying hen performance by dietary prebiotic chicory oligofructose and insulin. International Journal of Poultry Science, 4: 103 – 108.
DUNCAN, M. B. (1955). Multiple range and multiple F-tests. Biometrics, 11: 1 – 42.
GHASEMI, H. A., TAHMASBI, A. M., MOGHADDAM, G. H., MEHRI, M., ALIJANI, S., KASHEFI, E. and FASIFI, A. (2006). The effect of phytase and Saccharomyces cerevisiae (SC47) supplementation on performance, serum parameters, phosphorus and calcium retention of broiler chickens. International Journal of Poultry Science, 5(2): 162 – 168.
HAJATI, H. and REZAEL, M. (2010). The application of prebiotics in poultry production. International Journal of Poultry Science, 9(3): 298 – 304.
HUFF, G. R., HUFF, W. E., RATH, N. C. and TELLEZ, G. (2006). Limited treatment with B-1, 3/16- glucan improves production values of broiler chickens challenged with Escherichia coli. Poultry Sciences, 85: 613 – 618.
IJI, P. A. and TIVEY, D. R. (1999). The use of oligosaccharides in broiler diets. Pages: 193 – 201. In: Proceedings of the 12 th European Symposium on Poultry Nutrition, Veldhoven, The Netherlands WPSA Dutch Branch, Netherlands.
JÖZEFIAK, D., RUTKOWSKI, A., ENGBERG, R. M., HEJBERG, O. and JENSEN, B. B. (2004). Chicken caecal microflora as effected by dietary fibre of cereals and enzyme supplementation. Page 60. In: Proceedings of the 69 th Scientific Meeting of Polish Zootechnical Society, XVI Session of Polish WPSA Branch, United Kingdom.
KABIR, F. and HAQUE, T. (2010). Study on the production performance of Isa-Brown strain at Krishibid firm LTD, Trishal, Mymensingh. Bangladesh Research Publication Journal, 3(3): 1039 – 1044.
KHAN, S. H., HASAN, S., SARDAR, R. and ANJUM, M. A. (2008). Effects of dietary garlic powder on cholesterol concentration in native Desi laying hens. American Journal of Food Technology, 3: 207 – 213.
LEESON, S. and SUMMERS, J. D. (2005). Commercial Poultry Nutrition. Third Edition, University Books POBox 1326, Guelph, Ontario, USA.
MARTIN, P. A., BRADFORD G. D. and GOUS, R. M. (1994). A formula method of determining the dietary amino acid requirements of laying type pullets during their growing period. British Poultry Science, 35: 709 – 724.
NAHASHON, S. N., NAKAUE, H. S. and MIROSH, L. W. (1996). Performance of single comb white leghorn fed a diet supplemented with a live microbial during the growth and egg laying phases. Animal Feed Science Technology, 57(1-2): 25 – 38.
NAHASHON, S. N., NAKAUE, H. S., SNYDER, S. P. and MIROSH, L. W. (1994). Performance of single comb White Leghorn layers fed corn–soybean meal and barley–corn–soybean meal diets supplemented with a direct-fed microbial. Poultry Science, 73(11): 1712 – 1723.
PANDA, A. K., RAMA RAO S. V., REDDY, M. R. and PRAHARAJI, N. K. (2003). Production performance, serum/yolk cholesterol and immune competence of white leghorn layers as influenced by supplementation with probiotic. Tropical Animal Health Production, 35: 85 – 94.
SANTIN, E., MAIORKA, A., MACARI, M., GRECCO, M., SANCHEZ, J. C., OKADA, T. M. and MYASAKA, A. M. (2001). Performance and intestinal mucosa development of broiler chickens fed diets containing Saccharomyces cerevisiae cell wall. Journal of Applied Poultry Research, 10: 236 – 244.
SCHAFER, C. M., CORSIGLIA, C. M. MIRELES, A. and KOUTSOS, E. A. (2006). Turkey breeder hen age affects growth and systemic and intestinal inflammatory responses in female poults examined at different ages posthatch. Poultry Science, 85(10): 1755 – 1763.
TOUSSANT, M. J. and LATSHAW, J. D. (1999). Ovomucin contents and composition in chicken eggs with different interior quality. Journal of the Science of Food and Agriculture, 79(12): 1666 – 1670.
VARGAS, J. G., TOLEDO, R. S., ALBINO, L. F. T., ROSTANGO, H. S., OLIVEIVA, J. E. and CARVALHO, D. C. O. (2002). Characteristic as de frag der carte, submetidose antibiotics. In:XXIXI da SB, 200, Recife. ANais and Recife 2002, CDROM.
VERHEYEN, G. and DECUYPERE, E. (1991). Egg quality parameters in second and third laying years as a function of the moulting age, strain and moulting methods. Archiv fur Geflugelkunde, 55: 275 – 282.
YALCIN, S., ONBASILAR, E. E., REISLI, Z. and YALCIN, S. (2006). Effect of garlic powder on the performance, egg traits and blood parameters of laying hens. Journal of the Science of Food and Agriculture, 86: 1336 – 1339.
YASMEEN, F., MAHMOOD, S., HASSAN, M., AKHTAR, N. and YASEEN, M. 2008. Comparative productive performance and egg characteristics of pullets and spent layers. Pakistan Veterinary Journal, 28(1): 5 – 8.
YÖRÜK, M. A., GÜL, M., HAYIRLI, A. and MACIT, M. (2004). The effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens. Poultry Science, 83(1):84 – 88.
ZHANG A. W., LEE, B. D., LEE, S. K., LEE, K. W., AN, G. H., SONG, K. B. and LEE, C. H. (2005). Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poultry Science, 84(7): 1015 – 1021.







.jpg&w=3840&q=75)