INTRODUCTION
In China, Spain, Italy, Mexico and Peru, amongst other countries, the consumer demands chicken with yellow skin pigmentation. Since skin pigmentation is associated with health status and product freshness (Breithaupt 2007, Liu et al 2008, Hernandez-Velasco et al 2014), there is a largevolume of whole chicken or parts commercialized with the skin (Baker and Günther 2004). The inclusion level of dietary pigment for broiler chickens varies depending upon the company, region, and country.
Commercial competition and shorter grow-out periods require the use of high concentrations of dietary xanthophylls (XA), which may represent 8 to 10% of the diet cost. In Mexico, it is very typical to feed 80 ppm or more in the last 3 or 4 weeks of the grow-out (Muñoz-Díaz et al 2012), with XA consumption per bird between 240and 500 mg. These levels usually yield skin pigmentation levels of at least 22 skin yellowness units (b*) in live birds, and greater than 47 b* in cold carcasses, as measured in the lateral apterial pectoral area (Muñoz-Díaz et al 2012, Hernandez-Velasco et al 2014).
Amongst dietary carotenoids, in particular the XA (dihydroxycarotenoids) lutein and zeaxanthin are the components of major economic interest in Aztec marigold flower (Tagetes erecta) extract that are associated with natural yellow coloration in chicken skin and fat (Hadden et al 1999, Kotake-Nara and Nagao 2011). In additionto their pigmenting properties, XA are also antioxidants and provide natural UV protection, thus XA might be helpful to maintain healthy vision and skin. Therefore, inclusion of natural pigments in diets for poultry destined for human consumption may pose additional value to the health-conscious consumer (Baker and Günther 2004, Kijlstra et al 2012).
Skin pigmentation depends upon various factors such as: XA dose and consumption time, dietary nutritive quality, bird’s sex, bird health status, and slaughter processing conditions, among others (Britton et al 1998). From the diseases that influence skin pigmentation, coccidiosis is perhaps one of the most frequent and costly infections that affect the poultry industry globally (Ruff and Fuller 1975, Tyczkowski and Hamilton 1991). The severity of the coccidiosis effect on skin pigmentation will depend on the Eimeria strain and virulence, immune status of the bird, and other complicating factors. E. acervulina and E. maxima produce a significant reduction in plasma carotenoids (Ruff and Fuller 1975, Sharma and Fernando 1975, Ogbuokiri and Edgar 1986, Tyczkowski and Hamilton 1991). These infections are sometimes associated with lower body weight gain (BWG) that is a consequence of poor nutrient absorption due intestinal sloughing and villi shortening (Allen 1987). Plasma xanthophylls (PX) concentrations have proven to be sensitive indicators of the severity of Eimeria infections compared to other criteria such as live weight, macroscopic lesion scoring, and the number of oocysts shed in feces (Conway et al 1990).
Despite the economic impact of skin pigmentation in broiler chickens across various countries, and the fact that coccidiosis is the main infectious disease that affects it, there are very few studies, if any, that quantify the effect of Eimeria spp. infections on broiler skin pigmentation and how it may be influenced by bird’s sex. Therefore, the objectives of the current study were to evaluate the effect of Eimeria spp infection on XA absorption and deposition and ileal digestibility in broiler chickens, and to assess the recovery in skin pigmentation in broilers exposed to a mild coccidia infection.
MATERIAL AND METHODS
FACILITIES
The experiments were conducted at the Center for Research, Education, and Extension in Poultry Production (CEIEPAv) and the diagnostic laboratory of the Avian Medicine Department (DMZA) of the College of Veterinary
Medicine and Zootechnics (FMVZ), at the National Autonomous University of Mexico (UNAM).
EXPERIMENTAL BIRDS
Two experiments were conducted with sexed Ross 308 broiler chickens. The birds were separated by sex, and raised from 0 to 21 d of age in floor pens in an open-sided house. The experiments were conducted in accordance with the Guidelines of the Institutional Animal Care and Use Committee of the FMVZ, at the UNAM (CICUAE-FMVZ-UNAM). For both experiments, the birds were individually weighed at 21 d of age such that each pen had similar weight and weight distribution.
FEED
Diets were based on sorghum-soybean meal, and were formulated for three feeding phases: starter, 0-10 d; grower, 11-20 d; and finisher, 21-49 d of age. The diets contained meat and bone meal, and were designed to meet the nutrient specifications as recommended by the primary breeder (Aviagen 20071). Feed and water were provided ad libitum in a mash form. During the starter phase, all birds received 125 ppm of nicarbazine, and 100 ppm of sodium monensin during the grower phase, and only treatment one in the finisher phase in Experiment 1. In the finisher phase, all birds received 85 ppm of dietary XA from Aztec marigold flower.
EXPERIMENTAL DESIGN
Experiment 1. Four hundred and thirty two 21-d-old Ross308 sexed broiler chickens were randomly assigned to 4 treatments: 1) Non-infected control; 2) 8.32 x 104 sporulated Eimeria oocysts (SEO) per bird; 3) 17.82 x 104SEO perbird; and 4) 49.92 x 104 SEO per bird. Treatments contained 4 replications (2 replications of males and 2 of females) of 27 birds each. All birds received 85 ppm of dietary XA from Aztec marigold flower from 21 to 49 d of age.
Experiment 2. Four hundred 21-d-old Ross 308 sexedbroiler chickens were randomly assigned to 4 dietary treatments containing 4 replications (2 replications of males and 2 of females) of 25 birds each. Treatments were designed as follows: 1) Non-infected control + 85 ppm of dietary XA; 2) 8.32 x 104 SEO/bird + additional 23ppm of XA (108 ppm total); 3) 8.32 x 104 SEO/bird + additional 56 ppm of XA (141 ppm total); and 4) 8.32 x 104 SEO/bird + additional 77ppm of dietary XA (162 ppm total). At 28, 29, 38, and 39 d of age, all birds were treated with toltrazuril at 7mg/kg of body weight (BW) via drinking water. The infected birds (treatments 2, 3, and 4) received sodium monensin from 36 to 49 d of age. All birds received 85 ppm of XA from 21 to 36 d of age. The infected treatments received higher levels of dietary XA from 36 to 49 d of age, at the levels indicated above.
CHALLENGE
In both experiments, at 21 d of age, the birds from treatments 2, 3, and 4 received an inoculum containing vaccine strains of Eimeria acervulina (58%), E. maxima (20%), and E. tenella (22%) in 1 ml of sterile PBS via oral gavage. The concentration of the inoculum for each experiment was given as described above.
OOCYST COUNTS AND LESION SCORING
Prior to the beginning of the experiment, fecal samples from 5 birds/pen were collected to obtain a baseline measurement of Eimeria spp. infection. Coccidia oocysts were quantified by using the McMaster technique as previously described (Long and Rowell 1975). This procedure was performed at 14 d, 21 d, and weekly during the experimental period. Intestinal lesion scoring was conducted in 2 birds/pen on the same days as oocyst counts using the scoring system developed by Johnson and Reid (1970).
PLASMA XANTHOPHYLLS
Starting at 21 d, and then weekly for the duration of the study, 2 ml of blood was collected from the wing veins of 3 birds per pen and mixed with EDTA in a 10:1 proportion. The test tubes were covered with aluminum foil to protect the blood samples from light, and stored in refrigeration for further measurement of plasma XA according to the method reported by Allen (1987).
SKIN YELLOWNESS
Starting at 21 d, and then weekly for the duration of the study, skin yellowness units (b*) were determined in the right lateral apterial area of 10 birds/pen, using a reflectance colorimeter (Model CR-400, Minolta Corp., Ramsay, NJ.) (Muñoz-Díaz et al 2012).
GROWTH PERFORMANCE
All birds were weighed by pen at 21 d and 49 d of age. Feed intake was measured weekly to calculate BWG (g), feed consumption, pigment consumption, and feed conversion ratio (FCR).
APPARENT ILEAL PIGMENT DIGESTIBILITY
In Experiment 2, titanium dioxide was added to the diet at 0.2% and fed to the control and challenged birds from 28 to 35 d of age. At 35 d of age, 2 birds per pen were euthanized via CO2 asphyxiation and the ileal digesta was collected and frozen, freeze-dried, homogenised, and split into 2 subsamples for measurement of yellow XA by HPLC and titanium dioxide by spectrophotometry according to the methodology described by Ravindran et al (1999). A feed sample from control and challenged groups was collected and analysed for total XA and titanium dioxide.
STATISTICAL ANALYSIS
Growth performance, b*, and plasma xanthophyll concentration were analyzed by ANOVA for a 4 x 2 factorial arrangement, where the first factor was the coccidia infection at 4 levels, and the second factor was sex at 2 levels. Data for ileal digestibility was analyzed using a 2 x 2 factorial arrangement in which the first factor was the infection level (non-infected or infected), and the second factor was the sex (males or females). Treatment means were compared by Tukey’s multiple comparison procedure (P < 0.05).
Data for skin and plasma pigmentation was fitted to a multiple regression model in which the independent variables were the time of pigment consumption and the coccidia infection level; sex was used as an indicative variable for which 0 = females, and 1 = males (Neter et al 1985). Oocyst fecal count data and the intestinal lesion scores were analyzed using the non-parametric Kruskall-Wallis test. Treatment medians were compared by the Mann-Witney U test (Kuehl 2001).
RESULTS
EXPERIMENT 1
In Experiment 1, from 21 to 49 d of age, BWG in birds from the control group (2,427 g) was significantly greater than in birds from treatment 4 (2,239 g), which was challenged with the highest coccidia dose (P < 0.05). The best FCR was observed in the birds from the control treatment (1.96 kg:kg), which was significantly better from that observed in the treatment with the highest coccidia challenge (2.22 kg:kg). As expected, there was a significant effect of sex on growth performance. In males, BWG (2,538 g), feed consumption (5,110g) and pigment consumption (434mg) were significantly greater than in females (2,102 g, 4,589 g, 390 mg, respectively), while FCR was better in males (2.01 kg:kg) when compared to the females (2.18 kg:kg) (table 1).
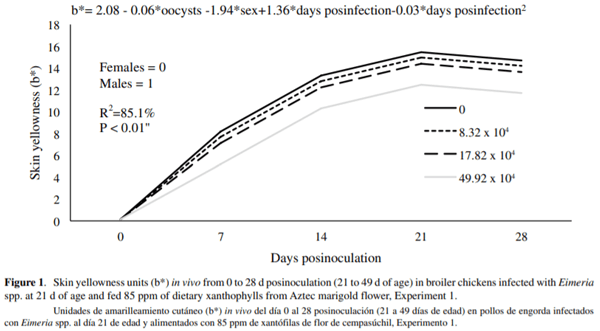
Between 0 and 28 d postinoculation (21 and 49 d of age), for every d that pigment was consumed, skin yellowness was increased by 1.36b* for both males and females. However, the rate of skin pigmentation was greater in female birds (1.94b*). For every 10,000 SEO that birds received, skin pigmentation decreased by 0.06b* (figure 1). This relationship can be described by the following equation: b*= 2.08 - 0.06*oocysts - 1.94*sex + 1.36*d post-infection - 0.03*d post-infection2; R2=85.1%; P < 0.01. Skin yellowness (SY) in live birds at 49 d of age was significantly greater (P < 0.05) in the control group (17.6b*) with respect to treatments 2, 3, and 4 (13.07, 11.9 y 13.8 b*). SY in males was significantly lower than in females (12.8 b* and 15.5 b*, respectively) (P < 0.05) (table 2).
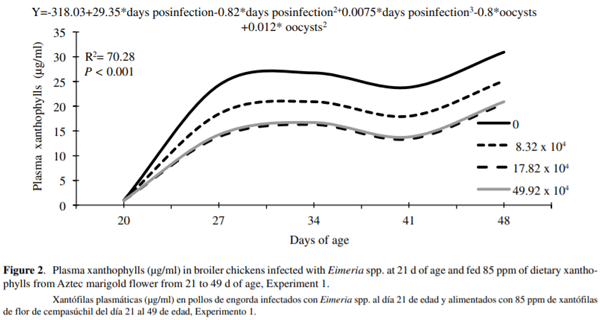
Concentration of PX was reduced by 0.8 µg/ml for every 10,000 SEO given to each bird. (figure 2). The effect of coccidia inoculation on PX concentration can be explained by the following equation: Y=-318.03+29.35*DI-0.82*DI2+0.0075*DI3-0.8*oei+0.012* SOE2; R2=70.28: P < 0.001, in which Y = PX concentration; DI = d post-infection; SEO = sporulated Eimeria spp. oocysts inoculated. Results for PX concentration are presented in table 2. Birds from the unchallenged control treatment had significantly greater PX concentration (39.7 µg/ml) compared to the challenged treatments (25.5, 19.9 and 25.6 µg/ml respectively). Sex had no significant effect on PX concentration, and there were no interactions between treatments (P < 0.05).
The oocyst count at the beginning of the study was negative for all treatments. After 21 d of age, the control treatment remained negative for oocysts. At 7 d post-inoculation, birds from treatment 4 had significantly greater oocyst counts medians (16.14 x 104 oocysts/g) with respect to treatments 2 or 3 (2.42 x 104 and 4.42 x 104 oocysts/g, respectively). At 14 d post-inoculation, there was a significant reduction in oocyst count in treatment 4 when compared to the previous week (3.45 x 104 oocysts/g). In this treatment, the oocyst count was lower than in treatment 3 (6.98 x 104 oocysts/g), but not different from treatment 2 (2.88 x 104 oocysts/g). At 21 d and 28 d post-inoculation, the oocyst counts were lower for treatment 2 (1.08 x 104 oocysts/g) with respect to treatment 4 (3.96 x 104 oocysts/g), but not different from treatment 3 (2.31x 104 oocysts/g) (P < 0.05). The median of intestinal lesion scores in treatment 1 was zero, whereas for treatments 2, 3, or 4, the median lesion score was 1+ or less, and no statistical difference was found between these treatments (P > 0.05), (data not shown).
EXPERIMENT 2
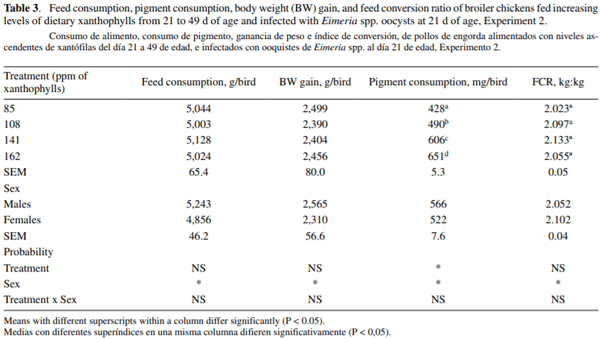
Growth performance was not significantly different between treatments at 49 d of age. However, males had significantly higher feed consumption (5,243 g), BWG (2,565 g), and better FCR (2.05 kg:kg) than females (4,856 g, 2,310 g, and 2.1 kg:kg, respectively) (P < 0.05). Pigment consumption was significantly greater in the males, and it differed across treatments according to the level of dietary XA. Birds from treatments 2, 3, and 4 received higher concentration of dietary XA from 35 to 49 d of age, and their XA consumption was 62, 178, and 223 mg per bird greater than that observed in the birds from the control treatment (table 3).
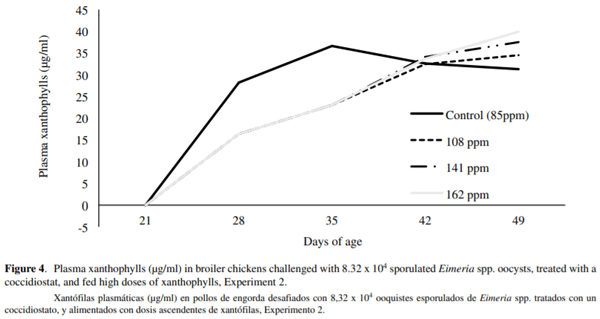
SY was not different between treatments at 49 d of age (figure 3). Female birds had significantly greater skin yellowness (23.72b*) than male birds (21.47b*). PX concentration was not different between treatments at the end of the study (figure 4), and no significant effect of bird’s sex was detected (P > 0.05).
Apparent ileal XA digestibility was significantly reduced in the birds challenged with coccidia (53.4% in males and 44.2% in females) in comparison with the control group (66.2% in males and 61.3% in females). No effect of bird’s sex was detected (P > 0.05).
DISCUSSION
The reduction in BWG and increase in FCR observed in the birds challenged with the highest coccidia dose is in agreement with previous research findings. Infection with E. acervulina and E. maxima detrimentally affected growth performance 2 to 3 weeks after inoculation (Hein 1968, Preston-Mafham and Sykes 1970). This effect is related to the damage caused by the replication of the parasite in the intestine (Allen and Danforth 1984) that leads to poor digestion and absorption of nutrients (Ruff and Fuller 1975). As expected, the birds that received lower coccidia challenge doses showed intermediate values for BWG and FCR, due to the degree of damage associated with the lower dose of infective oocysts (Stephens et al 1967). In 9-d-old birds infected with 105 E. acervulina oocysts, 6.7 x 103 E. maxima oocysts, and 104 E. tenella oocysts, there was a significant reduction in BW at 5 d post-inoculation (Conway et al 1993).
The rate of XA absorption is affected by coccidia infections and it might not recover to normal levels for up to 20 d post-infection. Absorption of other nutrients like glucose might not recover to normal rates before 14 d post-infection (Ruff and Wilkins 1980), thus birds may recover their BWG before they regain skin pigmentation after a coccidia infection. This could explain why birds from treatments 2 and 3 had better BWG than treatment 4. Nevertheless, none of the birds from the infected treatments achieved the same skin pigmentation observed in the non-challenged birds.
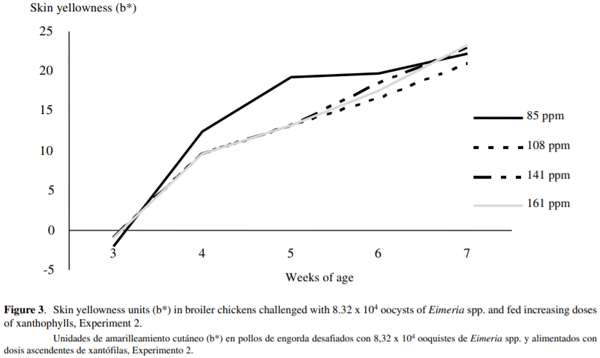
In Experiment 1, the reduction in skin pigmentation in birds challenged with coccidia was due to the reduction in the absorption of pigment, mainly caused by E. acervulina and E. maxima. Both Eimeria species produce villi sloughing and shortening in the intestinal regions where most of the pigment absorption occurs. On the other hand, E. tenella may induce a mild but continuous decrease in plasma carotenoids due to the blood loss associated with the damage in the cecal epithelium (McDougald and FitzCoy 2008). At the end of the study, SY was similar in all treatments, and the only difference was due to bird’s sex. The female birds had significantly higher skin pigmentation, even though the males showed greater pigment consumption. This finding is in agreement with the reports by Sirri et al (2010) and Muñoz-Díaz et al (2012) and it is attributed to higher deposition of subcutaneous fat in the females, which is related to genetic and hormonal factors (Le Bihan-Duval et al 1998, Rance et al 2002).
In both experiments, there was a reduction in PX concentration at 8 and 13 d post-infection, which has been reported previously (Juárez et al 2002). This might be due to the greater quantities of merozoites and gametocytes in the intestinal mucosa during this post-infection period (McDougald and Fitz-Coy 2008) that detrimentally influence XA absorption. The reduction in plasma carotenoids in birds infected with Eimeria spp. might also be related with a decline in the synthesis of Apo-I and Apo-III proteins by the enterocyte. These proteins have a major role in the transport of pigment to the liver and other tissues (Allen 1987). Furthermore, E. acervulina reduced the intestinal pH, which negatively affects pigment absorption (Kouwenhoven and van der Host 1969).
The highest PX concentration was found at 29 d of pigment consumption in all treatments. This is in agreement with Tepox (2013), who measured the highest PX concentrations after 28 d of consumption of dietary XA concentrations between 65 and 200ppm.
In Experiment 1, the number of oocysts shed through feces in the birds challenged with the highest infective dose (49.92 x 104 SOE) peaked at 7 d post-inoculation, and declined rapidly afterwards. A similar trend was observed in the intestinal lesions (gross white plaques) caused by E. acervulina for the same treatment. Such a reduction in oocyst shedding might be due to faster development of immunity in response to a more severe challenge. Therefore, the severity of intestinal lesions and oocyst shedding diminished rapidly. On the other hand, the birds challenged with lower oocyst doses required longer time to develop immunity, thus prolonging oocyst shedding. This could explain why the birds challenged with the intermediate oocyst doses had the highest oocyst shedding counts at 21 d post-infection.
In Experiment 1, there was no significant difference in the intestinal lesion scores, which were compatible with a subclinical infection according to the scale described by Johnson and Reid (1970). Although intestinal lesion scores have been used over the years to assess the degree of damage by coccidia infections both experimentally and under field situations, there seems to be no correlation between the degree of infection or the number of oocysts inoculated or shed through feces (Conway et al 1990, Kogut and Powell 1993).
Mild or subclinical Eimeria spp. infections are not easily detectable since they do not induce clear negative effects on growth performance. In countries where pigmented chicken is produced, subclinical coccidia infections are suspected only based on substandard skin pigmentation. Various studies have demonstrated that levels of plasma carotenoids and skin pigmentation are excellent response variables to evaluate coccidia infections due to the sensitivity of these measurements to the disease (Ruff and Fuller 1975, Sharma and Fernando 1975). In this study, these parameters were confirmed to be very good indicators of physiological intestinal integrity (Conway et al 1993).
Broiler chickens can reach adequate skin pigmentation levels if higher concentrations of dietary XA are fed for 14 d prior to slaughter age (Muñoz-Díaz et al 2012). In Experiment 2, even though the birds were infected with Eimeria spp., they were able to recover the expected SY after being treated with an anticoccidial drug, and fed with at least 62 mg of XA/bird from 35 to 49 d of age. It is possible that this effect might have been influenced by the high enterocyte turnover rate. During mild coccidia infections (1 x 105 E. acervulina oocysts per bird), the acute phase (4-8 d post-infection) is characterized by a sharp drop in absorption and deposition of pigment, and a recovery phase from 9 to 14 d post-infection (Sharma and Fernando 1975). Regeneration of intestinal epithelium occurs rapidly during mild coccidia infections and can be completed by 10 d post-infection (McDougald and Fitz-Coy 2008). Our results suggest that if the coccidia infection is controlled, and the birds received prompt anticoccidial treatment, absorption capacity and skin deposition of pigment may be fully recovered (Mathis et al 2004). In addition, feeding very high concentrations of dietary XA sometimes does not result in a significant increase of SY but results in a waste of XA.
No difference was detected in apparent ileal pigment digestibility between males and females, which is in agreement with previous research reported elsewhere (Tepox 2013). The results of the current study suggest that pigment is absorbed in a similar rate in males and females. However, pigment deposition seems to be more efficient in females. In the study reported herein, pigment digestibility was reduced by 15 percent in birds challenged with coccidia with respect to unchallenged birds. Although digestibility of carbohydrates, protein, and amino acids in chickens has been vastly evaluated (Yutste et al 1991, Ravindran et al 1999, Lemme et al 2004), to the author’s knowledge, pigment digestibility has not been reported before, especially as influenced by coccidia infections.
Pigmentation measurements could be helpful tools to determine the virulence of Eimeria species and strains. It would be necessary to conduct further studies to evaluate the effect of other Eimeria strains on these measurements to determine their limitations or benefits as compared to other criteria commonly used to determine the pathological effects of Eimeria infections such as oocyst counts and lesion scoring.
This article was originally published in Archivos de Medicina Veterinaria. 2016, vol.48, n.2, pp.199-207. ISSN 0301-732X. http://dx.doi.org/10.4067/S0301-732X2016000200010. This is an Open Access article distributed under a Creative Commons Attribution License. 


















.jpg&w=3840&q=75)