Effect of different levels of sunflower meal and multi-enzyme complex on performance, biochemical parameters and antioxidant status of laying hens
Adeola, O. & Cowieson, A.J., 2011. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89, 3189-3218.
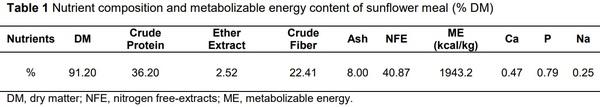
Alagawany, M., Faraj, M.R., Abd El-Hack, M.E. & Dhama, K. 2015. The practical application of sunflower meal in poultry nutrition. Adv. Anim. Vet. Sci. 3, 634-648.
Annison, G. & Choct, M., 1991. Anti-nutritive activities of cereal non-starch polysaccharides in broiler diets and strategies minimizing their effects. World Poult. Sci. J. 47, 232-242.
Association of Official Analytical Chemists (AOAC)., 2000. Official Methods of Analysis. Arlington, VA, USA.
Casartelli, E.M., Filardi, R.S., Junqueira, O.M., Laurentiz, A.C., Assuena, V. & Duarte, K.F., 2006. Sunflower meal in commercial layer diets formulated on total and digestible amino acids basis. Braz. J. Poult. Sci. 8,167-171.
Cowieson, A.J. & Adeola, O., 2005. Carbohydrase, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poult. Sci. 841, 860-1867. de Morais Oliveira, V.R., de Arruda, A.M.V., Silva, L.N.S., de Souza, J.B.F., de Queiroz, J.P.A.F., da Silva Melo, A., &
Holanda, J.S., 2016. Sunflower meal as a nutritional and economically viable substitute for soybean meal in diets for free-range laying hens. Anim. Feed Sci. Technol. 220, 103-108.
Deaton, J.W., McNaughton, J.L. & Burdick, D., 1979. High-fiber sunflower meal as a replacement for soybean meal in layer diets. Brit. Poult. Sci. 20,159-162.
Elkin, R.G. & Rogler J.C., 1990. Reduction of the cholesterol content of eggs by the oral administration of lovastatin to laying hens. J. Agr. Food Chem. 38, 1635-1641.
Grau, C.R. & Almquist, H.J., 1945. The value of sunflower seed protein. Proc. Soc. Exp. Biol. Med. 60, 373-374.
Jenkins, D.J.A., Wolever, T.M.S. & Jenkins, A., 1991. Specific types of colonic fermentation may raise low-density lipoprotein-cholesterol concentrations. Am. J. Clin. Nutr. 54, 141-147.
Karunajeewa, H., Than, S.H. & Abu-Serewa, S., 1989. Sunflower seed meal, sunflower oil and full-fat sunflower seeds, hulls and kernels for laying hens. Anim. Feed Sci. Technol. 26, 45-54.
Khan, S.H., Atif, M., Mukhtar, N., Rehman A. & Fareed, G., 2011. Effects of supplementation of multi-enzyme and multispecies probiotic on production performance, egg quality, cholesterol level and immune system in laying hens. J.
Appl. Anim. Res. 39, 386-398.
Khan, S.H., Sardar, R. & Siddique B., 2006. Influence of enzymes on performance of broilers fed sunflower-corn based diets. Pak. Vet. J. 26, 109-114.
Kocher, A., Chret, M., Porter, M.D. & Broz J., 2000. The effects of enzyme addition to broiler diets containing high concentrations of canola or sunflower meal. Poult. Sci. 79, 1767-1774.
Laudadio, V., Bastoni, E., Introna, M. & Tufarelli, V. 2013. Production of low-fiber sunflower (Helianthus annuus L.) meal by micronization and air classification processes. CyTA J. Food. 11, 398-403.
Laudadio, V., Ceci, E., Lastella, N.M.B. & Tufarelli, V., 2014a. Effect of feeding low-fiber fraction of air-classified sunflower (Helianthus annus L.) meal on laying hen productive performance and egg yolk cholesterol. Poult. Sci.
93, 2864-2869.
Laudadio, V., Ceci, E., Nahashon, S.N., Introna, M., Lastella, N.M.B. & Tufarelli, V., 2014c. Influence of substituting dietary soybean for air‐classified sunflower (Helianthus annuus L.) meal on egg production and steroid hormones in early‐phase laying hens. Reprod. Dom. Anim. 49, 158-163.
Laudadio, V., Introna, M., Lastella, N.M.B. & Tufarelli, V., 2014b. Feeding of low-fibre sunflower (Helianthus annus L.) meal as substitute of soybean meal in turkey rations: Effects on growth performance and meat quality. J. Poult.
Sci. 51, 185-190.
Laudadio, V., Ceci, E., Lastella, N.M.B., & Tufarelli, V., 2015. Dietary high-polyphenols extra-virgin olive oil is effective in reducing cholesterol content in eggs. Lipids Health Dis.14, 5.
Lebiedzinska, A., & Szefer, P., 2006. Vitamin B in grain and cereal-grain food, soy products and seeds. Food Chem. 95,
116–122.
Lee, K.W., Choi, Y.I., Moon, E.J., Oh, S.T. & Lee, H.H., 2014. Evaluation of dietary multiple enzyme preparation (Natuzyme) in laying hens. Asian-Australas. J. Anim. Sci. 27, 1749-1754.
National Research Council (NRC)., 1994. Nutrient Requirements of Poultry, 9th edition, National Academy Press.
Washington, D.C.
Pesti, G.M., & Miller, B.R., 1992. Animal Feed Formulation: Economic and Computer Applications. Nostrand Reinhold (Van), New York, NY.
Ravindran, V., 2013. Feed enzymes: The science, practice, and metabolic realities. J. Appl. Poult. Res. 22, 628-636.
Rezaei, M. & Hafezian, H., 2007. Use of different levels of high fiber sunflower meal in commercial Leghorn type layer diets. Int. J. Poult. Sci. 6, 431-433.
Rose, R.J., Coit, R.N. & Sell, J.L., 1972. Sunflower seed meal as a replacement for soybean meal protein in laying hen rations. Poult. Sci. 51, 960-967.
Salem, A.A., El-Anwer, E.M.M., Abo-Eita, E.M.M. & Namra, M.M.M., 2008. Productive and physiological performance of golden montazah male chickens as affected by feed restriction and avizyme supplementation. Egypt Poult. Sci.
28, 1137-1164.
SAS Institute, 2003. SAS/STAT User's Guide. Release 9.3 Ed. SAS Institute Inc., Cary, NC.
Sateri, S., Seidavi, A., Bouyeh, M., Neumann, P., Kutzler, M., Laudadio, V., F. Loperfido & Tufarelli, V., 2017. Effect of olive meal and supplemental enzymes on performance traits, blood biochemistry, humoral immunity response and caecal microbiota of broilers. S. Afr. J. Anim. Sci. 47, 804-812.
Satoh, K., 1978. Serum lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clin. Chim.
Acta 90, 37-43.
Senkoylu, N. & Dale, N., 1999. Sunflower meal in poultry diets: a review. World Poult. Sci. J. 55, 153-174.
Shi, S.R., Lu, J., Tong, H.B., Zou, J.M. & Wang, K.H., 2012. Effects of graded replacement of soybean meal by sunflower seed meal in laying hen diets on hen performance, egg quality, egg fatty acid composition, and cholesterol content. J. Appl. Poult. Res. 21, 367-374.
St-Onge, M.P., Farnworth E.R. & Jones, P.J.H., 2000. Consumption of fermented and nonfermented dairy roducts: effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 71, 674-681.
Tsuzuki, E.T., de Garcia, E.R.M., Murakami, A.E., Sakamoto, M.I. & Galli J.R., 2003. Utilization of sunflower seed in laying hen rations. Braz. J. Poult. Sci. 5, 179-182.
Tufarelli, V., Dario, M., & Laudadio, V., 2007. Effect of xylanase supplementation and particle-size on performance of guinea fowl broilers fed wheat-based diets. Int. J. Poult. Sci. 4, 302-307.
Ulbricht, T.L.V., & Southgate, D.A.T., 1991. Coronary heart disease: seven dietary factors. Lancet 338, 982-992.
Uwayjan, M.G., Azar, E.J. & Daghir, N.J., 1983. Sunflower seed in laying hen rations. Poult. Sci. 62, 1247-1253.
Vieira, S.L., Penz, A.M., Leboute, E.M. & Corteline, J., 1992. A nutritional evaluation of a high fibre sunflower meal. J.
Appl. Poult. Res. 1, 382-388.
Wolever, T.M.S., Spadafora, P.J. & Eshuis, H., 1991. Interaction between colonic acetate and propionate in humans.
Am. J. Clin. Nutr. 53, 681-687.
Wolever, T.M.S., Spadafora, P.J., Cunnane, S.C. & Pencharz, P.B., 1995. Propionate inhibits incorporation of colonic [1,
2-13C] acetate into plasma lipids in humans. Am. J. Clin. Nutr. 61, 1241-1247.
Yagi, K., 1984. Assay for blood plasma or serum. Method Enzymol. 105, 328-331.
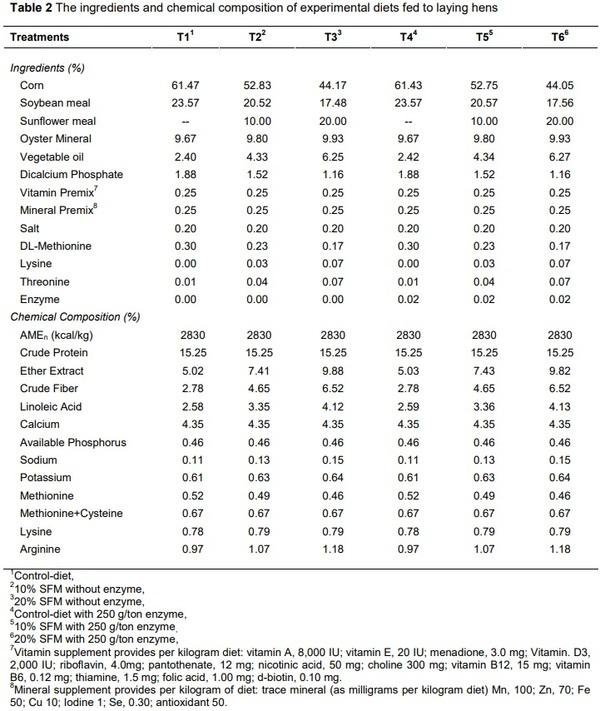
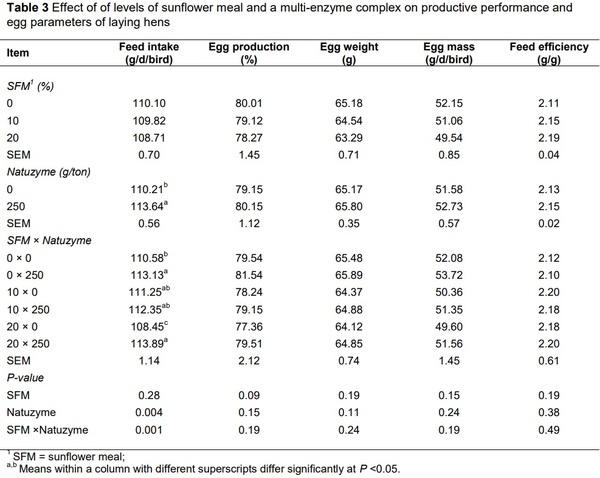
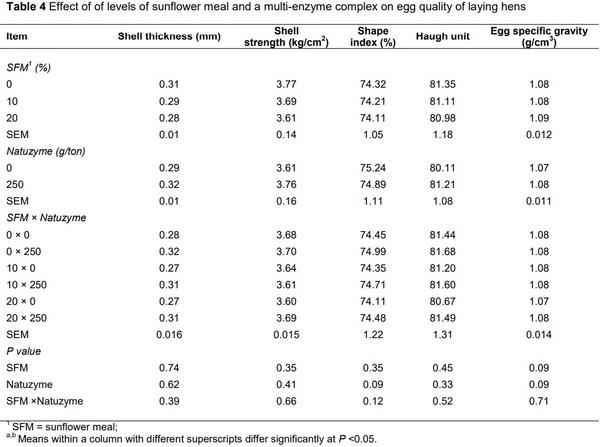
Thanks to the authors for an interesting publication, but I think that the practical priority in the matter belongs to the research center of Danisco AS. 25 years ago they formulated the working concept "Sunflower + Enzyme = Soy". The employees of my company supported this idea, because in Ukraine (the number 1 country in the world in the production of sunflower oil and sunflower meal), the cost of sunflower meal at that time was three times cheaper than soybean meal. We did not pay attention to the legal restrictions that set in Europe the maximum allowable level of input of sunflower meal in the feed of laying hens is about 13%. A few years later, all poultry farms switched to diets with 25% - 30% sunflower meal, without reducing productivity. Of course, it was necessary to add some lysine, but this did not really affect the final cost of the feed.
Kindly share the metabolizable energy prediction equation of sunflower meal for broiler and layer. Thanks.












.jpg&w=3840&q=75)