Effect of Aqueous Piliostigma Thonningii Leaf Extracts on the Heamatological and Serum Biochemical Indices of Broiler Chicken
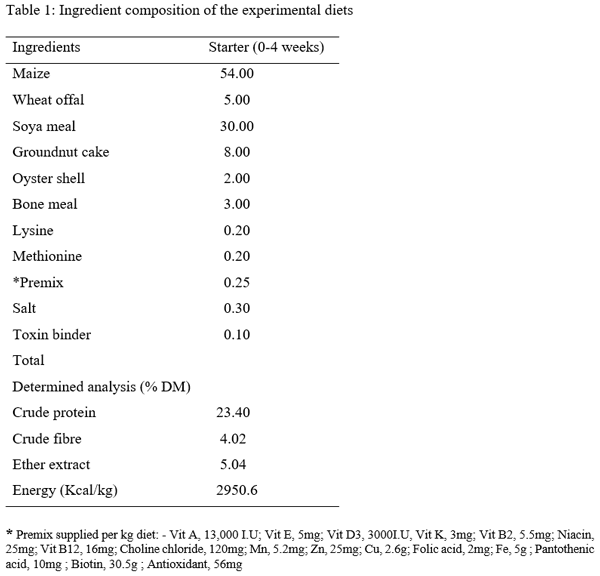
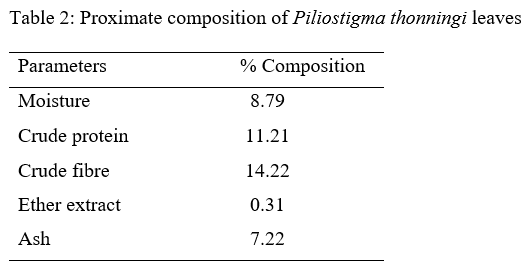
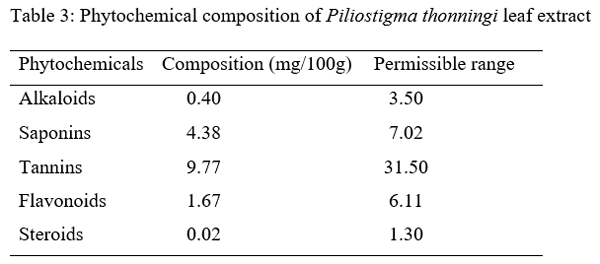
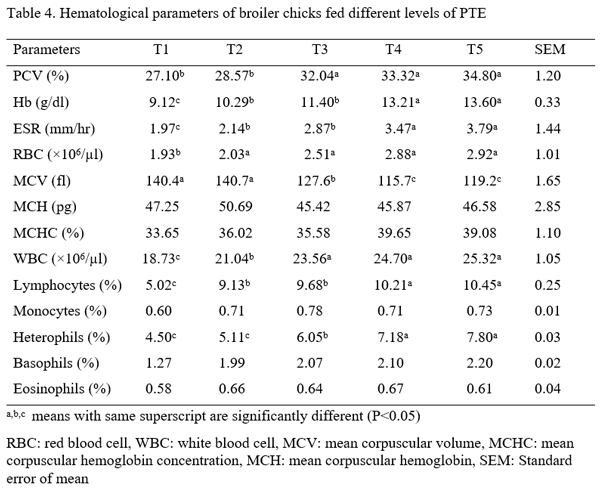
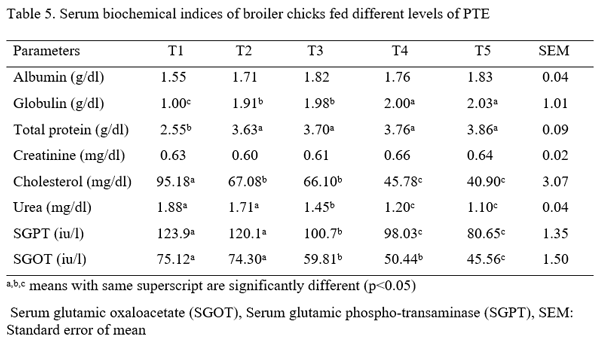
Three hundred one-day-old broiler chicks (Ross 308) were used to evaluate the effect of aqueous Piliostigma thonningii leaf extracts (PTE) on the some hematological and serum biochemical parameters of broiler chicken. The birds were randomly assigned to five treatments of four replicates consisting of 15 birds each in a completely randomized design. Birds in treatment 1 (T1) were given PTE at 0 % while T2, T3, T4 and T5 were fed PTE at 20ml, 40ml, 60ml and 80ml per liter of water. Feed and water were offered ad libitum throughout the experiment which lasted for 4 weeks. The hematological parameters examined are: pack cell volume (PCV), hemoglobin (Hb), red blood cells (RBC), erythrocyte sedimentation rate (ESR), white blood cells (WBC) and its differentials while those of serum biochemical parameters are: albumin, globulin, total protein, cholesterol, creatinine, urea, serum glutamic oxaloacetate (SGOT) and serum glutamic phospho-transaminase (SGPT). Result obtained showed that all the hematological parameters were significantly (P<0.05) different among the treatments. Albumin, globulin, total protein, cholesterol, urea, SGPT and SGOT values were significantly influenced (P<0.05) by the inclusion of PTE in the water of birds. Creatinine level were not significantly (p>0.05) different among the treatments. It could be concluded that PTE at levels up to 80 ml have no deleterious effect on the blood profile of birds.
Key words: Piliostigma thonningii leaf extract, hematology, broiler chicks
Akintomide, A.A., Joseph, O.G and Onibi, G.E. (2018). Hematology and serum biochemistry of cockerels fed diets containing neem leaf meal. Applied Tropical Agriculture. 23(1):12-16.
Abdi-Hachesoo, B., Talebi, A and Asri-Razaei, S. (2011). Comparative study on blood profiles indigenous and Ross-308 broiler breeders. Global Vet., 7:238-241.
Ayoola, A. A., Yusuf, A.O and Oki, D.G. (2016). Phytochemical screening and proximate analysis of Newbouldia laevis and Allium sativum. Nigerian Journal of Animal Science 1(2016):242-256.
Alagbe, J.O (2019). Growth performance and hemato-biochemical parameters of broiler chickens fed different levels of Parkia biglobosa leaf extracts. Academic Journal of Life Sciences 5(12): 107-115.
Adisa, R.M., Choudhary, E.A., Adenoye, G.A and Olorunsogo, O.O (2010). Hypoglycaemic and biochemical properties of Cnestis ferruginea. African Journal of Traditional Complementary Alternative Medicine. 7:185-194.
Azeez, O.I., Oyagbemi, A. A and Oyewale, J.O. (2009). Diurnal fluctuation in hematological parameters of the domestic fowl in the hot humid tropics. International Journal of Poultry Science. 8(3):247-251.
Alagbe, J.O (2019). Growth response and bacteria count of broiler starter given Delonix regia leaf extract as a natural alternative to antibiotics. Sumerianz Journal of Agriculture and Veterinary 2(9):76-81.
Akinpelu, D.A and Obuotor, E.M. (2000). Antibacterial activity of Piliostigma thonningii stem bark. Fitoterapia, 71(4): 442-443.
Aknladahunsi, A.A and Salawu, S.O. (2005). Phytochemical screening and nutrient-anti-nutrient composition of selected tropical green leafy vegetables. African Journal of Biotechnology, 4(6):497-501.
Alagbe, J.O (2017). Effect of miadasan as a dietary supplement on performance, carcass characteristics and blood profile of broiler chickens. Scholarly Journal of Agricultural Science 7(2), 27-33.
AOAC (2000). Official methods of analysis. 25th edition, Association of Official Analytical Chemists. Washington D.C, USA.
Boham, B.A and Kocipai, A. C. (1974). Flavonoids and condensed tannins from leaves of Hawaiian vaccinum vaticulatum and V. calycinium. Pacific Journal of Science. 48: 458-463.
Bello Oluwaseun., Zack Agbendeh and Adikwu Jacob G. (2013). Comparative studies of phytochemical screening of Ficus sycomorus linn stem bark extract and Piliostigma thonningii root extract. Asian Journal of Plant Science and Research 3(6):69-73.
Borges, L.P., Borges, V.C., Moro, A.V., Nogueira, C.W., Rocha, J.B.T and Zeni, G. (2005). Protective effect of diphenyl diselenide on acute liver damage induced by 2-nitropropane in rats. Toxicology, 210:1-8.
Butterworth, A.E (1999). Cell mediated damage in helminthes. Advanced Journal of Parasitology 23:143.
Cherkupally, R., Kota, S.R., Amballa, H and Reddy, B.N. (2017). In vitro antifungal potential of plant extracts against Fusarium oxysporum, Rhizoctonia solani and Macrophomina phaseolina. Annals of Plant Sciences, 6(9): 1676 – 1680.
Chineke, C.A., Ologun, A.G and Ikeobi, C.O.N. (2006). Hematological parameters in rabbit breeds and crosses in humid tropics. Pakistan Journal of Biological Sciences. 9(11):2102-2106.
Dey, B., Chowdhury, S.D., Bulbul, S.M and Chowdhury, B.L.D. (2011). Efficacy of neem leaf meal as a hypocholesterolemic dietary additive in laying pullets. Bangladesh Journal of Animal Science. 40 (2): 13-17.
Dabofunjo, O.P., Adebayo, A.H., Aliyu, R and Garba, I.H. (2013). The effects of ethanolic extract of Commiphora Africana on lipid profile of rats. International Journal of Pharmacology, 2(6):618-622.
Dabofunjo, O.P., Adebayo, A.H., Aliyu, R and Garba, I.H. (2012). The effects of methanolic extract of Philiostigma thonningii leaf on lipid profile of rats. International Journal of Pharmacology, 2(10): 501-508.
Duncan, D.B. (1955). Multiple range and multiple F-test. Biometrics 11(1):1-42.
Elagib, H.A.A and Ahmed, A.D.A. (2011). Comparative study on hematological values of blood of indigeneous chickens in Sudan. Asian Journal of Poultry Science. 5: 41-45.
Etim, N.N., Williams, M.E., Enyenihi, G.E., Udo, M.D and Offiong, E.E. (2013). Hematological parameters: indicators of the physiological status of farm animals. British Journal of Science 10(1):33-45.
Fakae, B.B., Cambell, A.M., Barrett, J., Scott, I.M., Teesdale-Spittle, P.H., Liebau, E and Brophy, P.M. (2000). Inhibition of gluthathione transferase from parasitic nematodes by extracts from traditional medicinal plants. Phytother Res, 14(1):630-634.
Harbone, J.D. (1973). Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hall, London, 279.
Gotoh, S., Takennako, O., Vatanabe, K., Kawamoto, R and Watanabe, T. (2001). Hematological values and parasitic fauna in free ranging Macaca hecki and the Macaca tonkeanai hecki hybrid group of Salawesi Island. Indonesia Primates 6:91.
Ibrahim Albokhadaim (2012). Hematological and some biochemical values of indigenous chickens in Al-Ahsa, Saudi Arabia during summer season. Asian Journal of Poultry Science 6(4):138-145.
Isaac, L.J., Abah, G., Akpan, B and Ekaette, I.U. (2013). Hematological properties of different breeds and sexes of rabbits (p.24-27). Proceedings of the 18th Annual Conference of Animal Science Association of Nigeria.
Igoli, J.O., Ogaji, O.G., Tor-Anyin. T.A and Igoli, N.P. (2005). Traditional medicine practice amongst the Igede people of Nigeria part II. African Journal of Traditional Complementary, (2):134-152.
Jain, N.C. (1986). Schalms veterinary hematology. 4th ed. Philadelphia: Lea and Febiger.
Ighodaro, I., Agunbiade, S.O., Omole, J.O and Kuti, O.A. (2012). Evaluation of the chemical, nutritional, antimicrobial and antioxidant vitamin profiles of Piliostigma thonningii leaves. Research Journal of Medicinal Plant 6(7):537-543.
Jimoh, F.O and Oladiji, A.T. (2005). Preliminary studies on Piliostigma thonningii seeds: proximate analysis, mineral composition and phytochemical screening. African Journal of Biotechnology, 4(2):1439-1442.
Lina Sernaite (2017). Plant extracts: antimicrobial and antifungal activity and appliance in plant protection (Review). Lithuanian Journal of Agriculture and Forestry, 3(4):58-66.
Kumar, V and Amit, K. (2010). Role of phytate and phytase. http//www.scribd.com/dietary.
Rates, S.M.K. (2001). Plant as source of drugs. Toxicon Journal, 29(2001): 603-613.
Talebi, A.S., Asri-Rezaei, S., Rozeh-Chai, R and Sahraei, R. (2005). Comparatives studies on hematological values of broiler strains (Ross, Cobb, Arbo-acres, Arian). International Journal of Poultry Science 4:573-579.
Norton, B.W (1994). The Nutritive value of tree legumes in: Forage tree legumes in Tropical Agriculture. Gutteride, R.C and Shelton H.M (Ed.) Cab International 177 (191):202-215.
Alagbe, J.O and Oluwafemi, R.A. (2019). Performance and hematological parameters of broilers given different levels of dried lemon grass and garlic extract. Research in: Agriculture and Veterinary Sciences, 3(1):102-111.
Odebiyi, A. and Sofowora, A.E. (1978). Phytochemical screening of Nigerian medicinal plant. Part III, Lloydia, 41, 234-246.
Olorede, B.R and Longe, O.G. (2000). Effect of replacing palm kernel cake with shear butter cake on quality characteristics, hematology and serum chemistry of laying hens. Nigerian Journal of Animal Production. 27:19-23.
Oloyede, O.B., Minari, J.B and Muhammad, N.O (2010). Evaluation of growth characteristics and hematological indices of broiler chicks fed raw and processed Bambara groundnut seed as a component of poultry feed. International Journal of Poultry Science. 9(7):625-655.
Obikaonu, H.O., Okoli, I.C., Opara, M.N., Okoro, M.O., Ogbuewu, I.P., Etuk, E.B and Udedibie, A.B.I. (2011). Hematological and serum biochemical indices of starter broilers fed neem (Azadirachta indica) leaf meal. Online Journal of Animal and Feed Research. 1(4): 150-154.
Ojewuyi, O.B., Ajiboye, T.O., Adebanjo, E.O., Balogun, A and Mohammed, A.O (2014). Proximate composition, phytochemical and mineral contents of young and mature Polyalthia longifolia leaves. Fountain Journal of Natural and Applied Sciences. 3(1): 10-19.
Soetan, K.O., Akinrinde, A.S and Ajibade, T.O. (2013). Preliminary studies on the hematological parameters of cockerels fed raw and processed guinea corn (Sorghum bicolor) Pg. 49-52. Proceedings of 38th Annual Conference of Nigerian Society of Animal Production.
Saleem, R., Ahmed, M., Ahmed, S.I and Azeem, M. (2005). Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia. Phytother Res. 19:881-884.
Ugwuene, M.C. (2011). Effect of dietary palm kernel meal for maize on the hematological and serum chemistry of broiler turkey. Nigerian Journal of Animal Science. 13: 93-103.
Vivian U. Oleforuh-Okoleh., Jude T. Ogunnupebi and Justice C. Iroka (2015). Asian Journal of Poultry Science. 9(4):242-249.
Van-Burden, T.P and Robinson, W.C. (1981). Formation of complexes between protein and tannic acid. Journal of Agriculture and Food Chemistry. 1:77.
WHO- World Health Organization (1992). Quality control methods for medicinal plant materials. Geneva.








.jpg&w=3840&q=75)