Dietary modulation of endogenous host defense peptide synthesis as an alternative approach to in-feed antibiotics
Traditionally, antibiotics are included in animal feed at subtherapeutic levels for growth promotion and disease prevention. However, recent links between in-feed antibiotics and a rise in antibiotic-resistant pathogens have led to a ban of all antibiotics in livestock production by the European Union in January 2006 and a removal of medically important antibiotics in animal feeds in the United States in January 2017. An urgent need arises for antibiotic alternatives capable of maintaining animal health and productivity without triggering antimicrobial resistance. Host defense peptides (HDP) are a critical component of the animal innate immune system with direct antimicrobial and immunomodulatory activities. While in-feed supplementation of recombinant or synthetic HDP appears to be effective in maintaining animal performance and alleviating clinical symptoms in the context of disease, dietary modulation of the synthesis of endogenous host defense peptides has emerged as a cost-effective, antibiotic-alternative approach to disease control and prevention. Several different classes of small molecule compounds have been found capable of promoting HDP synthesis. Among the most efficacious compounds are butyrate and vitamin D. Moreover, butyrate and vitamin D synergize with each other in enhancing HDP synthesis. This review will focus on the regulation of HDP synthesis by butyrate and vitamin D in humans, chickens, pigs, and cattle and argue for potential application of HDP-inducing compounds in antibiotic-free livestock production
Keywords: Host defense peptides, Antibiotic alternatives, Butyrate, Vitamin D, Antibiotics.
Alva-Murillo N, Medina-Estrada I, Baez-Magana M, Ochoa-Zarzosa A, LopezMeza JE. The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol Immunol 2015;68:445e55.
Alva-Murillo N, Ochoa-Zarzosa A, Lopez-Meza JE. Short chain fatty acids (propionic and hexanoic) decrease Staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet Microbiol 2012;155:324e31.
Balch DA, Rowland SJ. Volatile fatty acids and lactic acid in the rumen of dairy cows receiving a variety of diets. Br J Nutr 1957;11:288e98. Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 1990;70:567e90.
Biagi G, Piva A, Moschini M, Vezzali E, Roth FX. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J Anim Sci 2007;85:1184e91.
Bolognini D, Tobin AB, Milligan G, Moss CE. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol 2016;89:388e98.
Bommineni YR, Achanta M, Alexander J, Sunkara LT, Ritchey JW, Zhang G. A fowlicidin-1 analog protects mice from lethal infections induced by methicillin-resistant Staphylococcus aureus. Peptides 2010;31:1225e30.
Bommineni YR, Pham GH, Sunkara LT, Achanta M, Zhang G. Immune regulatory activities of fowlicidin-1, a cathelicidin host defense peptide. Mol Immunol 2014;59:55e63.
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 2005;3:238e50.
Campbell Y, Fantacone ML, Gombart AF. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur J Nutr 2012;51:899e907.
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011;17:1519e28.
Chen HP, Zhao YT, Zhao TC. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog 2015;20:35e47.
Choi MK, Le MT, Nguyen DT, Choi H, Kim W, Kim JH, et al. Genome-level identification, gene expression, and comparative analysis of porcine beta-defensin genes. BMC Genet 2012;13:98.
Choi S, Ingale S, Kim J, Park Y, Kwon I, Chae B. Effects of dietary supplementation with an antimicrobial peptide-P5 on growth performance, nutrient retention, excreta and intestinal microflora and intestinal morphology of broilers. Anim Feed Sci Technol 2013a;185:78e84.
Choi SC, Ingale SL, Kim JS, Park YK, Kwon IK, Chae BJ. An antimicrobial peptide-A3: effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Br Poult Sci 2013b;54:738e46.
Cuperus T, Coorens M, van Dijk A, Haagsman HP. Avian host defense peptides. Dev Comp Immunol 2013;41:352e69.
Cutler SA, Lonergan SM, Cornick N, Johnson AK, Stahl CH. Dietary inclusion of colicin e1 is effective in preventing postweaning diarrhea caused by F18- positive Escherichia coli in pigs. Antimicrob Agents Chemother 2007;51: 3830e5.
Daniel C, Schroder O, Zahn N, Gaschott T, Stein J. p38 MAPK signaling pathway is involved in butyrate-induced vitamin D receptor expression. Biochem Biophys Res Commun 2004;324:1220e6.
Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003;133: 2485Se93S.
Dhawan P, Wei R, Sun C, Gombart AF, Koeffler HP, Diamond G, et al. C/EBPalpha and the vitamin D receptor cooperate in the regulation of cathelicidin in lung epithelial cells. J Cell Physiol 2015;230:464e72.
Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol 2012;280:36e43.
Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci 2005;84:634e43.
Dimitrov V, White JH. Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol 2016;164:246e53.
Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol 2017;453:68e78.
Dixon BM, Barker T, McKinnon T, Cuomo J, Frei B, Borregaard N, et al. Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/ LL-37) and 25-hydroxyvitamin D levels in healthy adults. BMC Res Notes 2012;5:575.
Ernst RK, Guina T, Miller SI. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect 2001;3:1327e34.
Fang CL, Sun H, Wu J, Niu HH, Feng J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J Anim Physiol Anim Nutr (Berl) 2014;98:680e5.
Franklin MA, Mathew AG, Vickers JR, Clift RA. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J Anim Sci 2002;80:2904e10.
Fukae J, Amasaki Y, Yamashita Y, Bohgaki T, Yasuda S, Jodo S, et al. Butyrate suppresses tumor necrosis factor a production by regulating specific messenger RNA degradation mediated through a cis-acting AU-rich element. Arthritis Rheumatol 2005;52:2697e707.
Galfi P, Bokori J. Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Vet Hung 1990;38:3e17.
Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A 1994;91:11035e9.
Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Canc Ther 2003;2:151e63.
Goitsuka R, Chen CL, Benyon L, Asano Y, Kitamura D, Cooper MD. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc Natl Acad Sci U S A 2007;104:15063e8.
Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly upregulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005;19: 1067e77.
Gorka P, Kowalski ZM, Pietrzak P, Kotunia A, Jagusiak W, Holst JJ, et al. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J Dairy Sci 2011;94:5578e88.
Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr 2003;23:117e45.
Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 1996;238:325e32.
Guilloteau P, Savary G, Jaguelin-Peyrault Y, Rome V, Le Normand L, Zabielski R. Dietary sodium butyrate supplementation increases digestibility and pancreatic secretion in young milk-fed calves. J Dairy Sci 2010;93:5842e50.
Guilloteau P, Zabielski R, David JC, Blum JW, Morisset JA, Biernat M, et al. Sodiumbutyrate as a growth promoter in milk replacer formula for young calves1. J Dairy Sci 2009;92:1038e49.
Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem 2013;24:754e9.
Guo C, Sinnott B, Niu B, Lowry MB, Fantacone ML, Gombart AF. Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol Nutr Food Res 2014;58:528e36.
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008;27:104e19.
Han JE, Alvarez JA, Jones JL, Tangpricha V, Brown MA, Hao L, et al. Impact of highdose vitamin D3 on plasma free 25-hydroxyvitamin D concentrations and antimicrobial peptides in critically ill mechanically ventilated adults. Nutrition 2017;38:102e8.
Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 2012;10:243e54.
Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181:7090e9.
Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002;70:953e63.
Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 2013;9:761e8.
Hu Z, Guo Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim Feed Sci Technol 2007;132:240e9.
Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 2009;7:28.
Jiang W, Sunkara LT, Zeng X, Deng Z, Myers SM, Zhang G. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides 2013;50:129e38.
Jukes TH, Stokstad ELR, Taylor RR, Cunha TJ, Edwards HM, Meadows GB. Growthpromoting effect of aureomycin on pigs. Arch Biochem 1950;26:324e5.
Kallsen K, Andresen E, Heine H. Histone deacetylase (HDAC) 1 controls the expression of beta defensin 1 in human lung epithelial cells. PLoS One 2012;7, e50000.
Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 1997;9: 240e6.
Karlsson J, Carlsson G, Larne O, Andersson M, Putsep K. Vitamin D3 induces pro-LL37 expression in myeloid precursors from patients with severe congenital neutropenia. J Leukoc Biol 2008;84:1279e86.
Khoo AL, Chai LY, Koenen HJ, Oosting M, Steinmeyer A, Zuegel U, et al. Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 2011;55:294e300.
Kida Y, Shimizu T, Kuwano K. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol Immunol 2006;43: 1972e81.
Kotunia A, Wolinski J, Laubitz D, Jurkowska M, Rome V, Guilloteau P, et al. Effect of sodium butyrate on the small intestine. J Physiol Pharmacol 2004;55:59e68.
Kulkarni NN, Yi Z, Huehnken C, Agerberth B, Gudmundsson GH. Phenylbutyrate induces cathelicidin expression via the vitamin D receptor: linkage to inflammatory and growth factor cytokines pathways. Mol Immunol 2015;63:530e9.
Le Gall M, Gallois M, Seve B, Louveau I, Holst JJ, Oswald IP, et al. Comparative effect of orally administered sodium butyrate before or after weaning on growth and several indices of gastrointestinal biology of piglets. Br J Nutr 2009;102: 1285e96.
Lee WJ, Cha HW, Sohn MY, Lee SJ, Kim do W. Vitamin D increases expression of cathelicidin in cultured sebocytes. Arch Dermatol Res 2012;304:627e32.
Leeson S, Namkung H, Antongiovanni M, Lee EH. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult Sci 2005;84:1418e22.
Lhermie G, Grohn YT, Raboisson D. Addressing antimicrobial resistance: an overview of priority actions to prevent suboptimal antimicrobial use in food-animal production. Front Microbiol 2016;7:2114.
Lippolis JD, Reinhardt TA, Sacco RA, Nonnecke BJ, Nelson CD. Treatment of an intramammary bacterial infection with 25-hydroxyvitamin D(3). PLoS One 2011;6, e25479.
Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 2007;179:2060e3.
Lopez-Lopez N, Gonzalez-Curiel I, Castaneda-Delgado J, Montoya-Rosales A, Gandara-Jasso B, Enciso-Moreno JA, et al. Vitamin D supplementation promotes macrophages' anti-mycobacterial activity in type 2 diabetes mellitus patients with low vitamin D receptor expression. Microbes Infect 2014;16:755e61.
Lowry MB, Guo C, Borregaard N, Gombart AF. Regulation of the human cathelicidin antimicrobial peptide gene by 1alpha,25-dihydroxyvitamin D3 in primary immune cells. J Steroid Biochem Mol Biol 2014;143:183e91.
Lu L, Li SM, Zhang L, Liu XQ, Li DY, Zhao XL, et al. Expression of beta-defensins in intestines of chickens injected with vitamin D3 and lipopolysaccharide. Genet Mol Res 2015;14:3330e7.
Luecke RW, McMillen WN, Thorp Jr F. Effect of vitamin B12, animal protein factor and streptomycin on the growth of young pigs. Arch Biochem 1950;26:326e7.
Lynn DJ, Higgs R, Lloyd AT, O'Farrelly C, Herve-Grepinet V, Nys Y, et al. Avian betadefensin nomenclature: a community proposed update. Immunol Lett 2007;110:86e9.
Lyu W, Curtis AR, Sunkara LT, Zhang G. Transcriptional regulation of antimicrobial host defense peptides. Curr Protein Pept Sci 2015;16:672e9.
Mackenzie-Dyck S, Attah-Poku S, Juillard V, Babiuk LA, van Drunen Littel-van den Hurk S. The synthetic peptides bovine enteric beta-defensin (EBD), bovine neutrophil beta-defensin (BNBD) 9 and BNBD 3 are chemotactic for immature bovine dendritic cells. Vet Immunol Immunopathol 2011;143:87e107.
Mansour SC, Pena OM, Hancock RE. Host defense peptides: front-line immunomodulators. Trends Immunol 2014;35:443e50.
Mazzoni M, Le Gall M, De Filippi S, Minieri L, Trevisi P, Wolinski J, et al. Supplemental sodium butyrate stimulates different gastric cells in weaned pigs. J Nutr 2008;138:1426e31.
Meade KG, Cormican P, Narciandi F, Lloyd A, O'Farrelly C. Bovine beta-defensin gene family: opportunities to improve animal health? Physiol Genom 2014;46:17e28.
Merriman KE, Kweh MF, Powell JL, Lippolis JD, Nelson CD. Multiple beta-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol 2015;154:120e9.
Merriman KE, Poindexter MB, Kweh MF, Santos JE, Nelson CD. Intramammary 1,25- dihydroxyvitamin D3 treatment increases expression of host-defense genes in mammary immune cells of lactating dairy cattle. J Steroid Biochem Mol Biol 2017;173:33e41.
Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 2005;3:36e46.
Miraglia E, Nylen F, Johansson K, Arner E, Cebula M, Farmand S, et al. Entinostat upregulates the CAMP gene encoding LL-37 via activation of STAT3 and HIF-1alpha transcription factors. Sci Rep 2016;6:33274.
Moore PR, Evenson A, Luckey TD, McCoy E, Elvehjem CA, Hart EB. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J Biol Chem 1946;165:437e41.
Nancey S, Bienvenu J, Coffin B, Andre F, Descos L, Flourie B. Butyrate strongly in- hibits in vitro stimulated release of cytokines in blood. Dig Dis Sci 2002;47: 921e8.
Nelson CD, Reinhardt TA, Thacker TC, Beitz DC, Lippolis JD. Modulation of the bovine innate immune response by production of 1alpha,25-dihydroxyvitamin D(3) in bovine monocytes. J Dairy Sci 2010;93:1041e9.
Ochoa-Zarzosa A, Villarreal-Fernandez E, Cano-Camacho H, Lopez-Meza JE. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathog 2009;47:1e7.
Park K, Elias PM, Hupe M, Borkowski AW, Gallo RL, Shin KO, et al. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol 2013;133:1942e9.
Patil A, Hughes AL, Zhang G. Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genom 2004;20:1e11.
Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genom 2005;23:5e17.
Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 2006;4:529e36.
Ploger S, Stumpff F, Penner GB, Schulzke JD, Gabel G, Martens H, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 2012;1258:52e9.
Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn's disease: results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J 2015;3:294e302.
Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A 2006;103:9178e83.
Raqib R, Sarker P, Mily A, Alam NH, Arifuzzaman AS, Rekha RS, et al. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect Dis 2012;12:111.
Ravagnan G, De Filippis A, Carteni M, De Maria S, Cozza V, Petrazzuolo M, et al. Polydatin, a natural precursor of resveratrol, induces beta-defensin production and reduces inflammatory response. Inflammation 2013;36:26e34.
Rehman HU, Vahjen W, Awad WA, Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr 2007;61:319e35.
Rekha RS, Rao Muvva SS, Wan M, Raqib R, Bergman P, Brighenti S, et al. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy 2015;11: 1688e99.
Riggs MG, Whittaker RG, Neumann JR, Ingram VM. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 1977;268:462e4.
Robinson K, Deng Z, Hou Y, Zhang G. Regulation of the intestinal barrier function by host defense peptides. Front Vet Sci 2015;2:57. Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 1997;9:180e6.
Rodriguez-Lecompte JC, Yitbarek A, Cuperus T, Echeverry H, van Dijk A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult Sci 2016;95:2547e56.
S€ aemann MD, Bohmig GA, € Osterreicher CH, Burtscher H, Parolini O, Diakos C, et al. € Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J 2000;14: 2380e2.
Sang Y, Blecha F. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev 2008;9:227e35.
Sang Y, Blecha F. Porcine host defense peptides: expanding repertoire and functions. Dev Comp Immunol 2009;33:334e43.
Sarker P, Ahmed S, Tiash S, Rekha RS, Stromberg R, Andersson J, et al. Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One 2011;6, e20637.
Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 2006;118:509e19.
Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, et al. Histonedeacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol 2004;41:847e54.
Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 2008;128:816e24.
Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut 2003;52:735e41.
Scheppach W, Bartram HP, Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer 1995;31:1077e80.
Schogler A, Muster RJ, Kieninger E, Casaulta C, Tapparel C, Jung A, et al. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur Respir J 2016;47:520e30.
Schotterl S, Brennenstuhl H, Naumann U. Modulation of immune responses by histone deacetylase inhibitors. Crit Rev Oncog 2015;20:139e54.
Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, et al. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA 2002;99:2129e33.
Schwab M, Reynders V, Loitsch S, Steinhilber D, Schroder O, Stein J. The dietary histone deacetylase inhibitor sulforaphane induces human beta-defensin-2 in intestinal epithelial cells. Immunology 2008;125:241e51.
Schwab M, Reynders V, Shastri Y, Loitsch S, Stein J, Schroder O. Role of nuclear hormone receptors in butyrate-mediated up-regulation of the antimicrobial peptide cathelicidin in epithelial colorectal cells. Mol Immunol 2007;44: 2107e14.
Seal BS, Lillehoj HS, Donovan DM, Gay CG. Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim Health Res Rev 2013;14:78e87.
Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol 2005;6:551e7.
Shi J, Ross CR, Leto TL, Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47 phox. Proc Natl Acad Sci U S A 1996;93:6014e8.
Siciliano-Jones J, Murphy MR. Production of volatile fatty acids in the rumen and cecum-colon of steers as affected by forage:concentrate and forage physical form. J Dairy Sci 1989;72:485e92.
Stanton TB. A call for antibiotic alternatives research. Trends Microbiol 2013;21: 111e3. Steinmann J, Halldorsson S, Agerberth B, Gudmundsson GH. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother 2009;53: 5127e33.
Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One 2011;6, e27225.
Sunkara LT, Jiang W, Zhang G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One 2012;7, e49558.
Sunkara LT, Zeng X, Curtis AR, Zhang G. Cyclic AMP synergizes with butyrate in promoting beta-defensin 9 expression in chickens. Mol Immunol 2014;57: 171e80.
Svensson D, Nebel D, Nilsson BO. Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production. Inflamm Res 2016;65:25e32.
Takahashi D, Shukla SK, Prakash O, Zhang G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010;92:1236e41.
Tang XS, Fatufe AA, Yin YL, Tang ZR, Wang SP, Liu ZQ, et al. Dietary supplementation with recombinant lactoferrampin-lactoferricin improves growth performance and affects serum parameters in piglets. J Anim Vet Adv 2012;11:2548e55.
Tellez-Perez AD, Alva-Murillo N, Ochoa-Zarzosa A, Lopez-Meza JE. Cholecalciferol (vitamin D) differentially regulates antimicrobial peptide expression in bovine mammary epithelial cells: implications during Staphylococcus aureus internalization. Vet Microbiol 2012;160:91e8.
Termen S, Tollin M, Rodriguez E, Sveinsdottir SH, Johannesson B, Cederlund A, et al. PU.1 and bacterial metabolites regulate the human gene CAMP encoding antimicrobial peptide LL-37 in colon epithelial cells. Mol Immunol 2008;45:3947e55.
Thanner S, Drissner D, Walsh F. Antimicrobial resistance in agriculture. MBio 2016;7. e02227e02215.
Tian G, Liang X, Chen D, Mao X, Yu J, Zheng P, et al. Vitamin D3 supplementation alleviates rotavirus infection in pigs and IPEC-J2 cells via regulating the autophagy signaling pathway. J Steroid Biochem Mol Biol 2016;163:157e63.
van der Does AM, Bergman P, Agerberth B, Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol 2012;92:735e42.
Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel) 2014;7:545e94.
Wang S, Zeng X, Yang Q, Qiao S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci 2016;17.
Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25- dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004;173:2909e12.
Wen LF, He JG. Dose-response effects of an antimicrobial peptide, a cecropin hybrid, on growth performance, nutrient utilisation, bacterial counts in the digesta and intestinal morphology in broilers. Br J Nutr 2012;108:1756e63.
Wen Z-S, Lu J-J, Zou X-T. Effects of sodium butyrate on the intestinal morphology and DNA-binding activity of intestinal nuclear factor-kappaB in weanling pigs. J Anim Vet Adv 2012;11:814e21.
Whelehan CJ, Barry-Reidy A, Meade KG, Eckersall PD, Chapwanya A, Narciandi F, et al. Characterisation and expression profile of the bovine cathelicidin gene repertoire in mammary tissue. BMC Genom 2014;15:128.
Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl) 1996;74: 589e607.
Wu S, Zhang F, Huang Z, Liu H, Xie C, Zhang J, et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012;35:225e30.
Xiao H, Shao F, Wu M, Ren W, Xiong X, Tan B, et al. The application of antimicrobial peptides as growth and health promoters for swine. J Anim Sci Biotechnol 2015;6:19.
Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem 2006;281:2858e67.
Xiao Y, Hughes AL, Ando J, Matsuda Y, Cheng JF, Skinner-Noble D, et al. A genomewide screen identifies a single beta-defensin gene cluster in the chicken: implications for the origin and evolution of mammalian defensins. BMC Genom 2004;5:56.
Xiong H, Guo B, Gan Z, Song D, Lu Z, Yi H, et al. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci Rep 2016;6:27070.
Xiong X, Yang HS, Li L, Wang YF, Huang RL, Li FN, et al. Effects of antimicrobial peptides in nursery diets on growth performance of pigs reared on five different farms. Livest Sci 2014;167:206e10.
Xu H, Soruri A, Gieseler RK, Peters JH. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol 1993;38:535e40.
Yedery RD, Jerse AE. Augmentation of cationic antimicrobial peptide production with histone deacetylase inhibitors as a novel epigenetic therapy for bacterial infections. Antibiotics (Basel) 2015;4:44e61.
Yen JT, Nienaber JA, Hill DA, Pond WG. Potential contribution of absorbed volatile fatty acids to whole-animal energy requirement in conscious swine. J Anim Sci 1991;69:2001e12.
Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros 2007;6:403e10.
Yoon JH, Ingale SL, Kim JS, Kim KH, Lohakare J, Park YK, et al. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J Sci Food Agric 2013;93:587e92.
Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 2004;75:39e48.
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415: 389e95.
Zeng X, Sunkara LT, Jiang W, Bible M, Carter S, Ma X, et al. Induction of porcine host defense Peptide gene expression by short-chain Fatty acids and their analogs. PLoS One 2013;8, e72922.
Zhang G, Sunkara LT. Avian antimicrobial host defense peptides: from biology to therapeutic applications. Pharmaceuticals (Basel) 2014;7:220e47.
Zhang L, Lu L, Li S, Zhang G, Ouyang L, Robinson K, et al. 1,25-Dihydroxyvitamin-D3 induces avian beta-defensin gene expression in chickens. PLoS One 2016;11, e0154546.
Zhang WH, Jiang Y, Zhu QF, Gao F, Dai SF, Chen J, et al. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci 2011;52:292e301.
Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012;188:2127e35.
Zhao Y, Yu B, Mao X, He J, Huang Z, Zheng P, et al. Dietary vitamin D supplementation attenuates immune responses of pigs challenged with rotavirus potentially through the retinoic acid-inducible gene I signalling pathway. Br J Nutr 2014;112:381e9.
Zhou ZY, Packialakshmi B, Makkar SK, Dridi S, Rath NC. Effect of butyrate on immune response of a chicken macrophage cell line. Vet Immunol Immunopathol 2014;162:24e32.
Zhu K, Glaser R, Mrowietz U. Vitamin D(3) and analogues modulate the expression of CSF-1 and its receptor in human dendritic cells. Biochem Biophys Res Commun 2002;297:1211e7.
Dr. Zhang, I congratulate you on the published article. If I am not mistaken, I think that one of the problems of using the medicated diet with high doses of antimicrobials in the production of pork that is adopted in some countries is exactly the effect of these drugs in reducing the production of antimicrobial peptides that are naturally produced by animals due to the decreased activity of the microbiota.
I'm very interested in this article due to my 40 years of experience in Thai Livestock production, which tries to avoid using Antibiotics in animal farming. The good management practices and the ABO alternatives are most concerned. I would like to collect more information and learn more to educate and advise our customers.
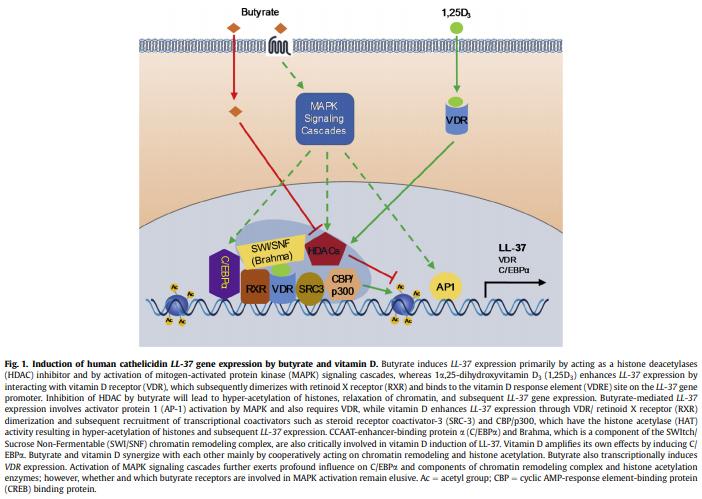










.jpg&w=3840&q=75)