Dietary Bacillus subtilis C-3102 Spores for Laying Hens: Egg Shell Quality and Brown Egg Color Score
Bacillus subtilis C-3102 spores are receiving increased attention as dietary direct-fed microbial (probiotic) performance enhancers for laying hens and breeders because as a feed supplement they typically are effective for improving egg shell thickness and brown egg shell color (in brown-egg strains), have high survivability through steam pelleting, and are economical (B.s. C-3102; CALSPORIN®, Calpis Co. Ltd, Tokyo, Japan).
This specific strain of beneficial microorganism was selected for efficacy by screening over 300 strains of beneficial bacteria, including several types of Bacillus spores. University, industry, and manufacturer’s trials have been conducted to evaluate performance of hens fed the supplement. This article will summarize some of these trial results, modes of action, and expected benefits when using B.s. C-3102 spores in poultry diets.
Modes of Action
Although some strains of Bacillus are capable of producing antibiotics or X-factors (e.g., bacitracin is obtained commercially from Bacillus licheniformis), and/or enzymes (e.g., Bacillus amyloliquefaciens is used commercially for starch hydrolysis), the main mode of action of B.s. C-3102 appears to be rapid oxygen consumption making the digesta more anaerobic which favors lactic acid producing bacteria.
Lactobacillus reuteri coating the intestinal tract, especially the crop and ceca, and other Lactobacilli are facultative anaerobes which proliferate under anaerobic conditions as shown by fresh fecal microbial assays. Lactobacilli produce lactic acid which inhibits pathogens such as Clostridium perfringens, enterotoxic E. coli, and Salmonella. These declines in pathogen counts are also confirmed by fresh fecal microbial assays (Figure 1).
Reduction in Clostridium perfringens numbers to safe levels within the intestinal tract is required for prevention of necrotic enteritis and associated mortality during times of clostridial challenge or stress. Prevention of Salmonella contamination of eggs has become extremely important because a high level of food safety is required by modern customers.
Egg Shell Quality
Calpis Co. Ltd received a U.S. patent (6,660,294) on December 9, 2003 for a “Poultry Eggshell Strengthening Composition” containing B.s. C-3102. The 1990s trial results with Dekalb hens cited in the patent application showed +5.2% average shell thickness improvement in Japan. With current strains, about +2 to +3% improvements in egg shell thickness are typical when using B.s. C-3102 in laying hen diets for demonstration field trials.
The mode of action by which dietary B.s. C-3102 enhances egg shell calcification is not completely known, but it is understood that laying hen femur osteoclasts, cells which resorb bone to provide about 7/8 of the calcium for egg shell formation, are more active than in unsupplemented control hens (Table 1). This may be due to more throughput of calcium as it seems unlikely that B.s. C-3102 would affect the carbonate portion of the calcium carbonate deposited in egg shells. During immune challenges, blood calcium level rises, possibly as a fever dampening mechanism and some of this circulating calcium may be lost from the body.
Therefore, it has been speculated that reducing the pathogenic challenges to the intestinal tract due to presence of dietary B.s. C-3102 spores and greater Lactobacillus numbers may allow a more steady blood calcium supply to the oviduct for shell formation. Or, more lactic acid production near the intestinal wall could conceivably improve limestone digestibility and calcium absorption. There are several possible mechanisms involving calcium metabolism that need to be explored.
Table 1.Effect of dietary Bacillus subtilis C-3102 on activity of osteoclasts, observed by microscope, in dissected femur (thighbone) of Dekalb TX laying hens 537-660 days old in Japan (August, 1996; Calpis Co. Ltd unpublished data)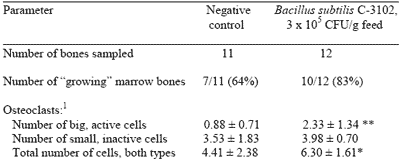
1Number of osteoclast cell types calculated as average of 5 fields per bone sample.
Hooge et al. (2005b) reported results of caged Hy-Line W-36 hen field trials in the U.S. midwest including microbe counts in fresh feces and either egg breaking strength or shell thickness (60 eggs/sampling) measurements. In experiment 1 (~140,000 hens/flock) at site 1 (57 wk old), egg breaking strength was 3.26 kg (5 weeks, 8 samplings) “before” and 3.33 kg (7 weeks, 9 samplings) "during" Bs C-3102 addition, with 3.20 kg expected from linear regression.
There was an estimated +0.13 kg improvement with B.s. C-3102. At site 2 (94 wk old, molted), egg breaking strength was 3.08 kg (8 weeks) "before" and 3.23 kg (7 weeks) "during" (P < 0.01), with 3.09 kg expected; estimated + 0.14 kg increase. In experiment 2 (~84,230 hens; 57 wk old; Figure 2), shell thicknesses were: "before" 0.321 mm (5 weeks), "during" 0.331 mm (7 weeks), "B.s. C-3102 removed" 0.313 mm (2 weeks), and "B.s. C- 3102 added" 0.324 mm (9 weeks).
There was a period of about 2 weeks in which the feed mill ran out of B.s. C-3102 product then was re-supplied. In some fecal samples prior to B.s. C-3102 supplementation, Lactobacilli/Total Anaerobes (CFU Log10/g) ratio was considerably below 50%, the desirable threshold level. It was concluded that diets containing B.s. C-3102 improved shell quality in these field trials.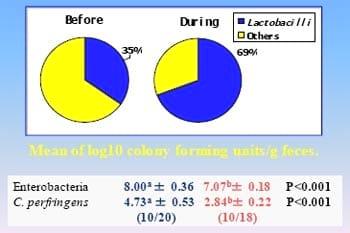
Figure 1. Lactobacilli / Total Anerobes (%) in Laying Hen Excreta Before and During Bacillus subtilis C-3102 Addition to the Diet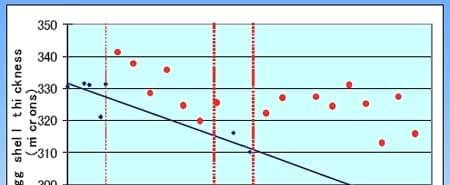
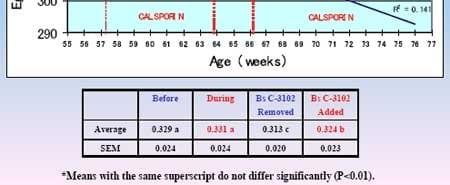
Figure 2. Laying Hen Field Trial -- Shell Thickness Hy-Line W-36 hens 57 wks age (before molting); 84,230 hens
Hooge et al. (2005a) described a small-scale experiment 1 (company A) in which 60 Hy- Line W-36 hens (59 weeks old) were used, and microbes in fresh feces and shell thickness were determined. Based on 4 samplings over 1 month, after a 1-week feeding adjustment period, shell thickness was 0.297 mm in the negative control vs 0.306 mm in the B.s. C-3102 group (+3.0%; P = 0.027).
Lactobacilli / Total Anaerobes increased from 44.4% in control to 56.4% in B.s. C-3102 group. In experiment 2 (company A), Hy-Line W-36 hens (43 weeks old) in a commercial flock were fed diets containing flax and soy oils (control vs B.s. C- 3102) for omega-3 eggs.
Based on 4 weekly egg samplings "before" and 5 samplings "during" B.s. C-3102 feeding (60 eggs/ sampling), shell thickness was 0.317 mm "before" and 0.323 mm "during" treatment (+1.9%; P = 0.034) despite advancing age of the hens.
In experiment 3 (company B), Lohmann hens (49 weeks old) in a commercial flock were fed diets "before" (3 weeks) and "during" B.s. C-3102 addition (8 weeks) resulting in respective shell thickness values of 0.339 mm vs 0.345 mm (+1.8%; P = 0.098; 60 eggs/sampling), in spite of age progression, and fecal Lactobacilli / Total Anaerobes increased from 34.7% to 69.3% (50% was desired theshold level). It was concluded that the B.s. C-3102 improved shell thickness.
Brown Egg Shell Color
Within the past 3 years, scientists discovered that B.s. C-3102 spores supplemented to laying hen diets can improve brown egg color of brown-egg strains usually within a matter of days. Typically, the initial darker brown colors of eggs produced by pullets gradually fade so that egg colors become lighter brown as hens age (Odabaşi et al., 2007).
However, brown egg color may also drop due to stressors such as high stocking density, handling, or loud noise (i.e., epinephrine release), to chemotherapeutic agents such as sulfonamides or nicarbazin, or to certain diseases such as Newcastle and infectious bronchitis (Butcher and Miles, 2003).
Therefore, a simple and economical intervention strategy involving dietary aerobic B.s. C-3102 spores (which vegetate, consume oxygen, and induce more favorable anaerobic conditions in digesta) to increase native Lactobacillus proliferation, decrease pathogens, and enhance egg shell quality and color would be welcome.
Importance of Brown Egg Color
Uniformity and intensity of brown egg color are important marketing considerations because excellent brown egg color may allow egg production to be extended, bring a premium price (e.g., in Japan and South Korea), and/or enhance marketing of natural and organic eggs (e.g., in the United States).
The brown egg sales as a proportion of total egg sales varies from country to country with highest numbers perhaps in China and South Korea at about 80% and in Puerto Rico at nearly 100%. Odabaşi et al. (2007) indicated that consumers prefer brown eggs over white eggs in several countries of the world, including United Kingdom, Italy, Portugal, Ireland, Southeast Asia, Australia, and New Zealand.
There may be a connection between egg shell quality and brown egg shell color. Joseph et al. (1999) reported that the darker brown the egg shell in four strains of broiler breeders, the higher the egg specific gravity, an indicator of shell quality. There was a small but highly significant positive correlation between brown color and egg specific gravity (i.e., they move in the same direction, or as egg shell color got darker egg specific gravity increased).
Brown Color Pigments
The mode of action by which B.s. C-3102 improves brown egg shell color is not known; however, the brown pigment involved originates from hemoglobin in red blood cells. Evanhoe (2006) reported that the brown pigment in egg shells is protoporphyrin whereas the blue pigment (e.g., in Araucana egg shells) or green pigment is oocyanin.
Butcher and Miles (2003) stated that during the last 3 or 4 hours of shell formation, epithelial cells lining the surface of the shell gland (uterus) synthesize and accumulate pigments for brown egg colour, mainly protoporphyrin-IX from the breakdown of hemoglobin in blood (Reucroft and Swain, 2006), along with some biliverdin-IX and its zinc chelate.
During the last 90 minutes or so of shell formation, the pigments are transferred to the protein-rich, viscous fluid secretion that becomes the cuticle. Cuticle with brown pigment can be removed with vinegar or sandpaper.
Whitening of Brown Eggs
It is well known that Nicarbazin, a broiler chemical coccidiostat, can cause whitening of brown eggs in broiler breeders due to crosscontamination of breeder feeds. Nicarbazin affects porphyrin metabolism in erythrocytes and the uterus, and markedly lowers uterine and egg shell porphyrin content (Schwartz et al., 1975). Nys et al. (1991) stated that in brown egg-laying strains with high or low incidences of shell whitening, whitening malfunction appeared to be due to impaired kinetics of porphyrin deposition because the amount of porphyrin deposited was normal.
Some hens stressed by means of cages which have open nest sites (nest hollows), as compared to enclosed nest sites, retained their eggs in the shell gland beyond the normal time of laying resulting in deposition of extra-cuticular calcium making brown eggs appear paler (Walker and Hughes, 1998).
A field survey of 2,206 eggs from Kadaknath breed hens in India showed 67.9% dark brown and 32.1% light brown eggs (Parmar et al., 2006), indicating possible stresses and room for improvement.
Brown Egg Color Field Trial (China 2006)
A preliminary field trial conducted at an egg farm in Asia in 2005 showed that B.s. C-3102 spores at the regular recommended dose (300,000 CFU/g feed) improved brown egg shell color within two weeks, as judged by Ghen Corporation brown color fan scores (1=light to 10=dark brown).
So next, a field trial (Miyazaki et al., 2007; Hooge, 2007a) was carried out with 63-weekold caged Lohmann Brown hens in 10 x 9,000-hen houses at Xiazhuang Egg Farm, Beijing, China in the spring of 2006 to compare regular vs B.s. C-3102 supplemented feeds.
Inclusion levels were 3.33x for 1 week, 2x for 2 weeks, and 1x for 4 weeks. Egg shell color was scored for 100 eggs per treatment collected each day from marked caged by the same person using the color fan. Results are shown in Figures 3 and 4, and Table 2.
Daily weighted brown egg color scores significantly (P < 0.001) increased during the first 2 weeks of feeding the B.s. C-3102 diet (6.29) compared to the pre-trial period (5.74) despite age progression. Compared to the pre-trial period, B.s. C-3102 supplementation increased egg counts in fan color score categories #7 (23.60% vs 14.11%; P < 0.001) and #8 (22.78% vs 17.98%; P < 0.001), and this shift was associated with a decreased egg count in #3 (5.03% vs 13.16; P < 0.001).
Lactobacilli / Total Anaerobes in fresh fecal samples increased from 56.4% pre-trial, a moderately high and healthy level for hens, to 75.20% after 43 days of B.s. C-3102 supplementation (P < 0.05). Enterobacteria (coliforms) decreased significantly (P < 0.01) from 5.02 down to 3.58 Log10 CFU/g fresh droppings due to the direct-fed microbial.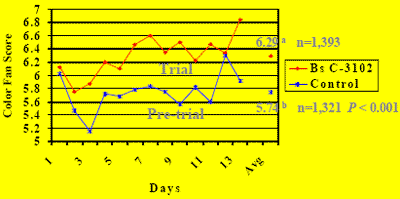
Figure 3. Brown Egg Shell Color Scores (China 2006)
Table 2.Means by Color Fan Score Category (China 2006)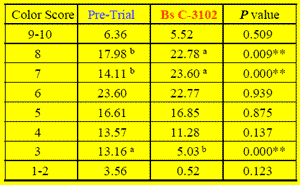
Broiler Breeders
In 2004 (unpublished data), a Brazilian company found that broiler breeders fed B.s. C-3102 diets had significantly improved total egg production to 65 weeks of age compared to those fed antibiotic control diets (185.03 vs 181.14; P < 0.05). Egg production percentages were not significantly different (87.85 vs 86.06%). All diets were pelleted. There were 3 flocks of 10,000 breeders each per treatment.
| Conclusion Spores of B.s. subtilis C-3102 have proven useful in the diets of laying hens to improve egg shell thickness or breaking strength and to increase brown egg color score of brown-egg strains compared to results for unsupplemented hens. When added to the diets of broiler breeder hens, B.s. subtilis C-3102 spores increased cumulative fertile egg production compared to results for hens fed diets containing an antibiotic. The proliferation of Lactobacillus species in the intestinal tract of B.s. subtilis C-3102 supplemented hens helps decrease the counts of intestinal pathogens such as Salmonella, E. coli, Campylobacter, and Clostridium perfringens based on fresh fecal microbial profiles. It is generally accepted that the presence of relatively low or normal levels of Clostridium perfringens or other pathogens in laying hens or breeders can spike up as a result of severe stressors such as high stocking density, disease challenge, feed and/or water deprivation, and so on, and cause morbidity, performance declines, or above-normal mortality. Brown egg strains are increasingly being used for production of free range, natural, or organic eggs, and these hens are often placed on litter with exposure to coccidia (coccidiosis), Clostridia (necrotic enteritis), and parasitic worms which may detrimentally affect feed digestibility and nutrient absorption resulting in adverse effects on shell quality, brown egg color, and egg production. In such cases, or simply as a preventive measure, supplementation of diets at around 50 weeks of age with B.s. subtilis C-3102 spores may improve these egg production parameters. |
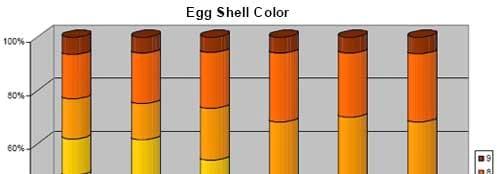
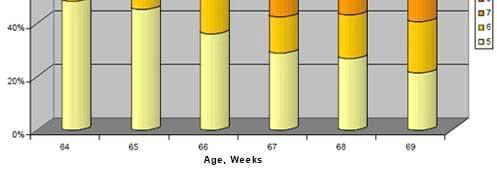
Figure 4. Brown egg color pre-trial 64-65 weeks and during Bacillus subtilis C-3102 feeding 66 weeks (3.33x), 67-68 weeks (2x), and 69 weeks (1x) in Beijing, China in 2006.
References
Butcher, G. D., and R. D. Miles. 2003. Factors causing poor pigmentation of brown-shelled eggs. University of Florida IFAS Extension Service, Gainesville, Florida, USA. Document VM94. 4 pp., accessed 6 February 2007.
Evanhoe, R. 2006. Chicken eggs. Chem. and Eng. News 84(34):49.
Hooge, D. M. 2007a. Bacillus subtilis spores improve brown egg color. World Poultry 3(23):14-15.
Hooge, D. M. 2007b. Dietary Bacillus subtilis C-3102 spores influence intestinal (excreta) populations of Lactobacilli, Clostridium perfringens, Enterobacteriaceae (coliforms), and Salmonella, and live performance of broiler chickens. Poult. Sci. 86(Suppl. 1):in press.
Hooge, D. M., M. Kato, and K. Nishimura. 2005a. Caged laying hen trials using dietary Bacillus subtilis C-3102 spores (Calsporin®) demonstrate improvements in egg shell thickness. Poult. Sci. 84(Suppl. 1): 88 (Abstr.)
Hooge, D. M., M. Kato, and K. Nishimura. 2005. Results of commercial caged laying hen field trials using dietary Bacillus subtilis C-3102 spores (Calsporin®) with emphasis on egg shell quality. Int. Poult. Sci. Forum Abstracts, #175, p. 42, Atlanta, Georgia, January 24-25.
Joseph, N. S., N. A. Robinson, R. A. Renema, and F. E. Robinson. 1999. Shell quality and color variation in broiler breeder eggs. J. Appl. Poult. Res. 8(1):70-74.
Miyazaki, H., S. Chou, M. Kato, and D. M. Hooge. 2007. Rapid and dramatic improvement in color intensity of brown egg shells from caged laying hens fed dietary CALSPORIN® (Bacillus subtilis C-3102 spores) in a commercial field trial in China. Int. Poult. Sci. Forum, January 22-23, Atlanta, Georgia, USA. Abstr. M70, p. 23.
Nys, Y., J. Zawadzki, J. Gautron, A. D. Mills. 1991. Whitening of brown-shelled eggs: mineral composition of uterine fluid and rate of protoporphyrin deposition. Poult. Sci. 70:1236-1245.
Odabaşi, A. Z., R. D. Miles, M. O. Balaban, and K. M. Portier. 2007. Changes in brown eggshell color as the hen ages. Poult. Sci. 86:356-363.
Parmar, S. N. S., M. S. Thakur, S. S. Tomar, and P. V. A. Pillai. 2006. Evaluation of egg quality traits in indigenous Kadaknath breed of poultry. Livestock Res. for Rural Dev. 18(9):(September)
Reucroft, S., and J. Swain. 2006. What determines the color of eggshells? Ask Dr. Knowledge, The Boston (Massachusetts) Globe, September 18. 1 page., accessed 6 February 2007.
Schwartz, S., B. D. Stephenson, D. H. Sarkar, M. R. Bracho. 1975. Red, white, and blue eggs as models of porphyrin and heme metabolism. Annals New York Acad. Sci. 244: 570-590.
Walker, A. W., and B. O. Hughes. 1998. Egg shell colour is affected by laying cage design. Br. Poult. Sci. 39(5):696-699.
Thank very much for the information. Could you recommend what is the brand of the one?
Why some paragraph under the heading of (broiler breeder) were pasted? I had roughly read before, interesting about the relationship b/t egg shell color intensity or uniformity and hatchability. Now I can not recall, I want to see again and please do something for that. Does it mean that the facts mentioned before are wrong? Please explain.
Thank you.

Nicarbazin main for the prevention of chicken cercal coccidiosis (Imperia) and the reactor type, giant, poisoned, E.brunetti (intestinal coccidiosis). According to tests, medication within 48h after infection coccidiosis, coccidia can effectively inhibit the development, if the drug later than 72h, the effect is significantly reduced









.jpg&w=3840&q=75)