Introduction
Certain mushrooms have been reported to offer benefits that include fast growth, improved nutrition, resistance to and protection from pathogens. Proximate and phytochemical analysis has shown that they contain bioactive compounds that function as good feed supplements and medicines to treat certain parasitic diseases and to improve wound healing.
Various drugs are available for the prevention or control of coccidiosis in chickens 4,5. Coccidiosis is an acute to chronic infection caused by protozoal parasites of the genus Eimeria, which multiply in the intestinal mucosa of chickens and produce severe tissue damage, resulting in bloody diarrhoea, reduced growth, weight loss, blood loss and increased susceptibility to other pathogens.
In Eimeria tenella infection, the parasites are confined to the caecum, as opposed to the other Eimeria species of chickens (E. necatrix, E. acervulina, E. brunetti, E. hagani, E. maxima, E. mivati, E. mitis and E. praecox), which infest the anterior, middle and lower parts of the small intestine. Reproduction of the parasites results in the formation of unsporulated oocysts 4–6. The unsporulated oocysts are shed in the faeces, and can infect other susceptible birds by ingestion of contaminated litter, feed and water or via mechanical carriers such as poultry house equipment, clothing, footwear, insects, other animals, wild birds and dust.
The Houghton strain of E. tenella (H strain) was isolated in the United Kingdom in about 1949 from a field case of caecal coccidiosis in chickens and maintained at the Houghton Poultry Research Station (HPRS), Houghton, and thereafter at the Institute for Animal Health (IAH) at Compton, UK, and has at various times been provided to other institutions and groups carrying out coccidiosis research on fowls 6. The characteristics of the parasite include its ubiquitous presence in the field, high virulence, and ease of handling in the laboratory due to the high rate of replication, ease of recovery of oocysts from the blind-ended caecal pouches, excellent sporulation of the oocysts and robustness of the sporozoites 6. Houghton (H) strain of E. tenella is fully pathogenic 6. A dose of 20 000 E. tenella oocysts (H strain) was sufficient to cause substantial mortality and reduction in weight gain 6. A comparison of the H strain of E. tenella and various laboratory and field strains revealed no significant differences in pathogenicity 6. Young chicks of about 4 weeks of age are most susceptible and the infection is characterised by acute onset of the 1st clinical sign, namely bloody droppings resulting from the invasion of the caecal mucosal epithelium by 2nd generation merozoites.
Early emphasis in the control of coccidiosis in poultry was placed on strict hygienic measures combined with preventive medication using sulphonamides such as sulphadimidine and sulphaquinoxaline, and other drugs as they became available, including amprolium (a thiamine analogue), diaveridine (a pyrimidine), ionophores (polyether antibiotics such as monensin, salinomycin and narasin), nitrofuran and furazolidone, robenidine (a guanidine derivative) and quinolones 12. Many poultry flocks currently receive preventive medication and curative treatment to control coccidiosis. Despite these control measures, coccidiosis continues to be a major constraint for efficient production of poultry in Nigeria, possibly owing to resistant strains and complexity of the parasites. Some drugs may kill the parasites (coccidiocides) but others only arrest the development of the parasites (coccidiostats).
In tropical Africa, particularly Nigeria, most species of wild mushroom (both edible and medicinal) are collected from the fields and marketed by women and children for human consumption and as medicines to improve health. Ganoderma lucidum, for instance, is a well-known species of medicinal mushroom in Chinese medicine, where it is cultivated, processed and sold as dried and whole or as powders, capsules, or tablets to treat certain diseases of the respiratory and gastrointestinal tracts, and for immunomodulation in humans 19,20,25. In China alone more than 700 medicinal products with mushroom as the main ingredient are commercially available, and according to statistics at least 106 medicinal products contain Ganoderma, 43Cordyceps, and 7 shiitake mushrooms 25. These natural health-promoting fungi are known to possess medicinally active polysaccharides 25. They are also known to be very rich in proteins, crude fibre, potassium, phosphorus, calcium, iron, manganese, zinc, B-complex vitamins, thiamine, riboflavin, niacin, biotin and essential amino acids, as well as a low level of unsaturated linoleic acid, essential for good health.
The need to explore locally available alternative additives or healthy food supplements and drugs to promote animal health and production, especially for control of major economically important diseases like coccidiosis in poultry, cannot be over-emphasised. Many of the anti-coccidial drugs are expensive, and resistance to some of the drugs has been reported 29–30. Ganoderma lucidum contains bioactive compounds or polysaccharides and resins that are known to kill parasites and to improve healing of wounds. It is used as a food supplement or as medicine to improve various parameters of health and immune functions in humans 32–34. The objective of this study was to determine whether the aqueous extract of wild G. lucidum can reduce E. tenella oocysts output in infected broilers, improve body weight gain and mitigate haematological changes that may occur.
Materials and Methods
Study site
The study was conducted at the Federal College of Animal Health and Production Technology, National Veterinary Research Institute in Vom, Plateau State, Nigeria.
Experimental birds
One hundred and twenty day-old Ross broilers were obtained on 15 August, 2006 from a hatchery in Jos, Plateau State, Nigeria. The birds were randomly distributed into 6 treatment groups (A–F) of 20 chicks each in wire cages, each measuring 80 × 100 cm.
Preparation of aqueous extract of Ganoderma lucidum
Wild Ganaderma lucidum with red open caps were harvested from wooden logs and tree stumps on farm land in Vom, Plateau State, Nigeria. They were washed in distilled water, sun-dried, ground to powder using a mortar and pestle and then blended using a Corona grinder (Landers & CIA, SA). The mushroom powder was again sun-dried for 3 h and then stored in plastic polythene bags and kept at room temperature until required for use. A 20 % weight to volume solution of the G. lucidumwas prepared by soaking in hot water boiled at 100 °C for 3 h, bringing the concentration to 200 mg/m . The solution was sieved, solid matter discarded and the filtrate allowed to cool to room temperature before use. A standard anticoccidial drug (amprolium) was also used at a concentration of 200 mg/m to compare its efficacy to that of G. lucidum.
Experimental infection and treatments
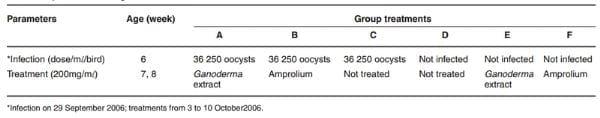
The birds in group A, B and C were infected with Eimeria tenella (Houghton strain) at the rate of 36 250 sporulated oocysts/m per bird using an insulin syringe introduced directly into the crop of each bird at 6 weeks of age between 08:30 and 10:30, on 29 September 2006. By day 6 post-infection (PI) they were treated with 200 mg/m of either G. lucidum aqueous extract or amprolium in drinking water given ad libitum for 7 days consecutively (Table 1).
Table 1: Experimental design of infection with Eimeria tenella and treatment of chicks.
Determination of weight and feed to gain ratio
Body weight gain of the broilers was monitored weekly using a weighing balance (made in China by Hana) every morning prior to feeding. The feed:gain ratio per bird/group was determined, where feed:gain per bird = total feed consumption by the birds in a cage divided by weight gain of surviving birds + weight gain of dead birds in the cage. The group with the highest value indicates evidence of depression of feed intake due to infection with E. tenella 7. The broiler mash contained maize, groundnut cake, wheat chaff, rice bran, fishmeal, bone-meal, limestone and premix, giving about 22 % crude protein and 2800 Kcal/kg metabolisable energy. The feeders and drinkers were washed daily using boiling water to reduce the risk of contamination.
Determination of packed cell volume
Two ml of blood were collected from the wing vein of each bird by venipuncture using sterile syringes and needles (1/bird) and the blood was immediately transferred into a set of sterile tubes containing anticoagulant, disodium-salt of ethylene diamine tetra-acetic acid (EDTA) for determination of packed cell volume (PCV). PCV was determined using the microhaematocrit method 11. The blood samples were collected weekly between 08:30 and 10:30 at 2, 4, 6 and 8 weeks of age, and the tests were conducted within 2 h of collecting the blood.
Collection of faecal samples and laboratory examination
The faeces of the broilers were collected daily in polythene bags from day-old to 10 weeks of age for parasitological examination. E. tenella oocyst output was also measured and expressed per gram faeces using a McMaster counting chamber 21. Faeces from each group were thoroughly mixed in plastic bottles using a spatula. One gram of the faecal sample was placed in a sterile bottle and homogenised by mixing with 1 m of flotation sodium chloride (NaCl) salt solution to make a suspension that was then mixed with 9 m of the salt solution, sieved in gauze wire mesh or muslin, the solid matter discarded and the filtrate collected in clean sterile plastic tubes filled to the brim and a cover slip was placed on top taking care to exclude air bubbles. The bottles were allowed to stand upright for 15 min to enable coccidia oocysts to float to the cover slip before examination under a light microscope at ×10 and ×40 magnifications. A portion of the positive sample only was used to fill the McMaster counting chamber and allowed to stand for about 15 min to enable oocysts to float and settle at the top of the chamber to facilitate identification and counting of the oocysts under the microscope using a differential counter. Absolute numbers of coccidia oocysts counted per m of the solution were recorded.
Statistical analysis
Feed:gain ratio per bird/group was determined 7. Duncan’s multiple range test was used to separate the means that were significantly different . Statistical analysis of variance was carried out 27.
Results
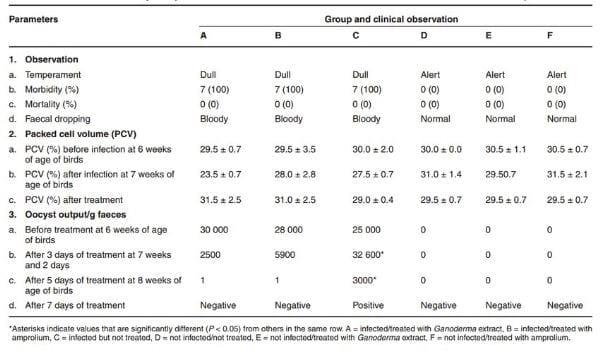
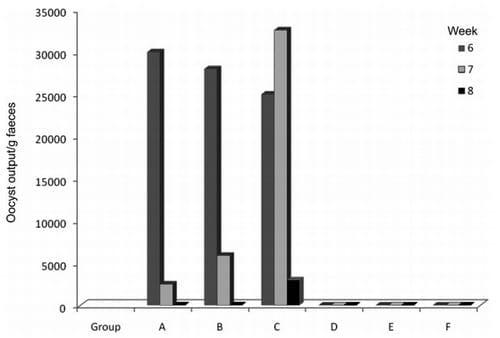
On day 4 post-infection (PI) all the birds in the infected groups A, B and C appeared dull and weak and had reduced appetite. By day 5 PI their faeces became bloody and watery and Eimeria tenella oocysts were detected in their faeces (Table 2), indicating that infection of the broilers with the E. tenellawas successful. Prior to treatment of the birds the oocyst output was 30 000 oocysts/g faeces (group A), 28 000 oocysts/g faeces (group B), and 25 000 ooccysts/g faeces (group C) (Fig. 1). The faeces of uninfected groups D, E, F were not bloody and were free of coccidial oocysts (Table 2). On the 3rd day after treatment the oocysts detected in the Ganoderma lucidum-treated group (A) had reduced significantly in number (2500 oocysts/g faeces) compared to the amproliumtreated group (B) (5900 oocysts/g faeces). Group C showed a significant increase in oocysts released (32 600 oocysts/g faeces) (Fig. 1). By day 5 after treatment, the oocysts released in A and B had reduced to just 1 oocyst/g faeces and by day 7 the birds in these groups were negative, while group C continued to discharge E. tenella oocysts up to day 17 post-infection. The uninfected control groups D, E and F did not pass any oocysts during the entire period of the experiment. No mortality occurred in any of the groups before the end of the experiment, but the caeca of the infected broilers were severely haemorrhagic (Table 2).
Table 2:Clinical features and oocyst output of broilers infected with Eimeria tenella and treated with Ganoderma lucidumaqueous extract.
Fig. 1: Faecal oocyst output of broilers: Group A infected/treated with Ganoderma extract, B infected/treated with amprolium, C infected/not treated, D not infected/treated with Ganoderma extract, E not infected/treated with amprolium, F not infected/not treated.
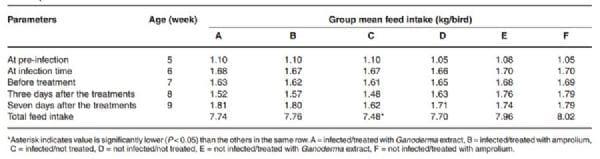
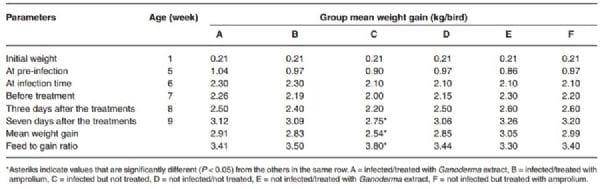
The results further showed that at 7 to 8 weeks after infection with E. tenella, the feed intake of birds in all the infected groups dropped from 1.6 kg to 1.5 kg, but this was followed by a compensatory increase in feed intake in group A and B (1.8 kg, each) after treatment at 9 weeks (Table 3). The feed intake was lower (1.6 kg) in group C (infected but not treated) and the feed to gain ratio was significantly higher (3.8) compared to all the other groups, B (3.5), D (3.4), A (3.4), F (3.4), E (3.3) (Table 4). The total feed intake in group C was significantly lower (P < 0.05). The mean weight gain of the birds in group C was also significantly lower (2.54 kg/bird) (Table 4). Haematological analysis showed a slight drop in PCV in groups A (23.5 % ± 0.7), B (28.0 % ± 2.8) and C (27.5 % ± 0.7) at 7 weeks of age (1 week PI), and the birds voided bloody diarrhoea between 4 and 6 days PI (Table 2). There was no bloody diarrhoea in groups D, E and F and the PCV of the birds in these groups remained higher (29.5 % ± 0.7 and higher).
Table 3: Group mean feed intake (kg) of broilers infected with Eimeria tenella and then treated with Ganoderma lucidum aqueous extract and amprolium.
Table 4: Group mean weight gain (kg/bird) of broilers infected with Eimeria tenella and then treated with Ganoderma lucidum aqueous extract and amprolium
Discussion
In this study, the broilers were successfully infected with H strain of Eimeria tenella as demonstrated by the fact that all the infected birds (100 %) showed clinical signs of weakness, reduced appetite, and bloody diarrhoea, and oocysts were present in their faeces by the 5th day post-infection. There was a significant reduction (P < 0.05) in faecal oocyst output in birds that were treated with either aqueous extract of G. lucidum or amprolium. There was also a significant reduction in the weight gain of the infected but untreated birds compared to those that were infected and treated, which showed improved weight gain. Their feed:gain ratio was also better (3.4) than the birds that were infected but not treated, which had the highest (3.8) feed to gain ratio. In other earlier studies, a significant reduction in body weight occurred in broilers infected with a dose of 10 000 sporulated oocysts of E. tenella 7.
The high feed:gain ratio (3.8) observed in the infected, untreated birds provides evidence of depression of feed intake due to infection with E. tenella. The highest feed:gain value reported was 1.61 in broilers that were infected with E. tenella which resulted in significant reduction in the body weight 7. However, no significant effect of infection on body weight gain was reported in a study in which birds were infected with 3500 or 5000 sporulated oocysts per bird of the H strain of E. tenella 10, although inoculation with 1000 to 3000 sporulated oocysts was said to be sufficient to cause bloody faeces and other signs of infection 22. It is, however, generally accepted that body weight gain is a sensitive but variable measurement for coccidiosis and efficacy of anticoccidial treatment 7,10, although high levels of coccidia oocyst inoculum may be needed to achieve measurable suppression of body weight gain in infected and untreated birds 7. Other factors such as starvation, immunosuppression, age and genetic predisposition of the birds may also be involved in the occurrence and severity of avian coccidiosis 30,31. The results in terms of feed to gain ratio, body weight and use of fungi to suppress oocysts of E. tenella H strain in broilers observed in this study compared well with those of other studies 7,10.
Among the reasons why the medicated broilers gained weight better than the non-medicated birds could be that the aqueous extract of G. lucidum, like amprolium may kill or prevent development of E. tenella and so in the absence of further development the broilers improved in weight gain better than those that were infected but not treated. It is also possible that the extract and amprolium stimulate appetite, so the broilers that were treated with them ate more and improved in weight gain better than those not treated. The broilers that were not infected but treated with either amprolium or Ganoderma performed even better in terms of weight gain than those that were infected and treated. It appears that this wild mushroom contains compounds that are active against E. tenella. The mushroom was found to be non-toxic in animal toxicity studies and in humans, even when used at high therapeutic doses 14,15,19. Bioactive compounds or polysaccharides are known to play vital roles in enhancing health; they block colonisation of the intestine by pathogens, thereby improving their elimination from the body 10,13,16. Some biologically active compounds or organic acids, resins, and glycosides which include steroid and triterpenoid saponins are known to have therapeutic uses against microbes and parasites 2,8,13,15. The mushrooms used in this study were found to contain these compounds.
Although the ranges of normal PCV values measured in these broilers were wide (23.5–32.5 %), the decline observed in Groups A, B and C may be attributed to coccidial infection. According to some authors, PCV may be sensitive to or affected by coccidiosis 7,24. A mean PCV of 19.0 % at 6 days post-inoculation was observed to be the minimum for survival in birds 7. In this experiment, there was no mortality and the minimum PCV recorded was 23.5 % post-inoculation. Wide ranges of normal haematological values were reported by other authors 17,23,26. Fluctuations in the haematological values of avian blood are known to be a normal phenomenon and in most instances the variations may depend on the physiological state of the birds 18.
Other studies have shown that some mushrooms have polysaccharides that play a role in stimulating the activities of many interdependent cell types such as T and B-lymphocytes, macrophages, and natural killer (NK) cells, inducing production and secretion of cytokines and complement 13. Other mushrooms (e.g. Fraxinella, Boletus and Lactarius spp.) have also been reported to prevent intestinal coccidiosis in poultry 1,13,14,28. Other authors reported that some mushrooms contain chemical substances that enhance the immune response and control certain parasitic and viral diseases 3,13,25,32–34. However, the active principles and the mechanisms of action of these mushrooms have not been fully elucidated, and should be the subject of future studies.
Conclusions
This experiment confirmed that infection of broilers with Eimeria tenella causes bloody diarrhoea as a result of damage to the intestinal mucosa leading to depression of feed intake and loss of body weight. Treatments with either Ganoderma lucidum or amprolium resulted in amelioration of clinical signs of bloody diarrhoea and reduction of faecal oocyst count. It also improved feed intake and weight gain. The treatments did not adversely affect PCV in E. tenella-infected and non-infected broilers. The results confirmed the virulence of Houghton strain of E. tenella and the effectiveness of amprolium against E. tenella.
Acknowledgements
The authors wish to acknowledge and dedicate this work to the memory of our dear colleague, the late Dr L.O Mgbojikwe who passed away in a ghastly motor accident. We also acknowledge the following for their assistance; Drs M.S Ahmed, A.O Olabode, Tai Cole, B.O Akanbi, Musa Usman, J. Kamani, J.K Tanko and Mr Samson Edokpolo, J.A Edache, D.S Gbise and Noel Dus, as well as Messrs B.O Oguntayo and T. Agnes, all of the National Veterinary Research Institute, Vom.
References
1. Adejumo T O, Awosanya O B 2005 Proximate and mineral composition of four edible mushroom species from the south western Nigeria. African Journal of Biotechnology 4: 1084–1088
2. Anon. 2006 Cultivation, utilization and medicinal effects of Ganoderma lucidum in Malaysia. Online at: http://www.canited.com/reishi97d-9.htm (accessed 30 August 2006)
3. Anon. 2007 Mushrooms may be active against fowl parasite.THISDAY11(4280): 36
4. Canning E W, Anwar M A 1968 Studies on meiotic division in coccidial parasites. Journal of Protozoology 15: 290–298
5. Chapman H D 1989 The sensitivity of field isolates of Eimeria tenella to anticoccidial drugs in chickens. Research in Veterinary Science 47: 125–128
6. Chapman H D, Shirley M W 2003 The Houghton strain of Eimeria tenella: a review of the type strain selected for genome sequencing. Avian Pathology 31: 115–127
7. Conway D P, Sasai K, Gaafar S M, Smothers C D 1993 Effects of different levels of oocysts inocula of Eimeria acervulina, E. tenella and E. maxima on plasma constituents, packed cell volume, lesion scores and performance in chickens. Avian Diseases 37: 118–123
8. Dei H K, Rose S P, Mackenzie A M 2007 Shea nut (Vitellaria paradoxa) meal as a feed ingredient for poultry. World’s Poultry Science Journal 63 : 611–624
9. Duncan D B, 1955 Mutiple range and multiple F tests. Biometry 11: 1–42
10. Elmusharaf M A, Bautista V, Nollet L, Beynen A C 2006 Effect of a mannanoligosaccharide preparation on Eimeria tenella infection in broiler chickens. International Journal of Poultry Science 5: 583–588
11. Esievo K A N, Saror D I 1992 A laboratory manual in veterinary clinical pathology (1st edn). Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria
12. Guneratine J R M, Gard D L 1991 A comparison of three continuous and four shuttle anticoccidial programme. Poultry Science 70: 1888–1894
13. Guo F C, Savelkoul H F J, Kwakkel R P, Williams B A, Verstegen M W A 2003 Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World’s Poultry Science Journal 59: 427–440
14. Harkonen M 1998 Uses of mushrooms by Finns and Karelians. International Journal of Circumpolar Health 57: 40–55
15. Hobbs C 1995 Medicinal mushroom. Botanica Press, Santa Cruz
16. Hughes D H, Lynch D L, Somers G F 1958 Chromatographic identification of the amino acids and carbohydrates in cultivated mushroom. Journal of Agriculture and Food Chemistry 6: 850–853
17. Iheukwuemere F C, Abu A H, Ameh M 2006 Effect of human menopausal gonadotropin on haematological and serum biochemical parameters of Nigerian indigenous chickens. Proceedings of the 20th Annual National Conference of Farm Management Association of Nigeria, Forestry Research Institute, Jos, Nigeria, 18–21 September 2006: 483–486
18. Islam M S, Lucky N S, Islam M R, Ahad A, Das B R, Rahman M M, Siddiu M S I 2004 Haematological parameters of Fayoumi, Assil and local chickens reared in Sylhet region in Bangladesh. International Journal of Poultry Science 3: 144–147
19. James M 2002 Reish mushroom extract and immune support. Dynamic Chiropractice 20: 1–5
20. Jong S C, Birminghan J M 1992 Medicinal benefits of the mushroom, Ganoderma. Applied Microbiology 37: 101–134
21. Long P L, Powell J G 1958 Counting oocysts of chicken coccidia. Laboratory Practice 7: 515
22. McDougald L R 2003 Coccidiosis. In Saif Y M, Barnes H J, Fadly A M, Glisson J R, McDougald L R, Swayne D E (eds) Poultry diseases. Iowa State University Press, Ames: 947–991
23. Mhatre A J, Joshi V G 1993 Effect of different temperature regimens on some haematological parameters in broilers. Indian Veterinary Journal 70: 915–920
24. Natt M P, Herrick C A 1955 The effect of caecal coccidiosis on the blood cells of the domestic fowl: a comparison of the changes in the erythrocyte count resulting from haemorrhage in the infected and mechanically bled birds; the use of haematocrit value as an index of the severity of the haemorrhage resulting from the infection. Poultry Science 34: 1100–1106
25. Oei P 2003 Benefits of mushrooms. InMushroom cultivation (3rd edn). Technical Centre for Agricultural and Rural Cooperation (CTA), Backhuys, Leiden: 1–7
26. Ogbe A O, Adeyefa C A O, Joshua R A 2003 Growth rate and haematological parameters of broiler chickens vaccinated with IBD (Gumboro) vaccines exposed to different handling temperature. Journal of Science and Technology Research 2: 36–38
27. Olawuyi J F 1996 Biostatistics: a foundation course in health sciences (1st edn). University College Hospital, Tunji Alabi Printing, Total Garden, Ibadan, Nigeria: 1–221
28. Pang F H, Xie M Q, Ling H H 2000 The investigation of immune-modulators tested for the results on the control of a coccidial infection. Chinese Journal of Veterinary Parasitology 8(3): 1–3
29. Peek H W, Landman W J M 2003 Resistance handling temperature. Journal of Science and Technology Research 2: 36–38 27. Olawuyi J F 1996 Biostatistics: a foundation course in health sciences (1st edn). University College Hospital, Tunji Alabi Printing, Total Garden, badan, Nigeria: 1–221
28. Pang F H, Xie M Q, Ling H H 2000 The investigation of immune-modulators tested for the results on the control of a coccidial infection. Chinese Journal of Veterinary Parasitology 8(3): 1–3
29. Peek H W, Landman W J M 2003 Resistance Chinese medicinal mushroom: biomarker responses in a controlled human supplementation study. British Journal of Nutrition 91: 263–269
33. Wasser S P 2002 Medicinal mushrooms, as source of antitumor and immune-modulating polysaccharides. Applied Microbiology and Biotechnology 6: 258–274
34. Zakhary J W, Taiseer M, Abo-Bakr A, El-Mahdy, Tabey S A 1983 Chemical composition of wild mushrooms collected from Alexandria, Egypt. Food Chemistry 11: 31–41.








.jpg&w=3840&q=75)