I. INTRODUCTION
Over the past few decades the meat, egg and milk sectors have faced the need to reduce the routine use of antibiotics in animal production, and the high incidence of food poisoning associated with animal product consumption. Approximately 130,000 tonnes of antibiotics were used in 2013 worldwide, with 75% of this in animals (Hughes, 2019). Up to 90% of these antibiotics can be excreted into the environment via urine and faeces, and approximately 400 resistance markers against 25 antibiotics can be found in chick caecal bacteria (Van Boeckel et al, 2019). Globally, around 700,000 human deaths per annum are attributed to antibiotic resistance and this is predicted to increase to 10 million by 2050 (FAO, 2019). With rising concern about the development of antibiotic resistance in human health, regulators, consumers and retailers have led the drive to reduce the sub-therapeutic use of antibiotics in animal feeds to zero. Endemic disease is re-emerging, adding costs to animal production systems and driving the need for alternative non-antibiotic interventions.
Food poisoning continues to be a problem across the world, with salmonellosis cases now increasing in many countries. Non-typhoidal salmonellosis is reported to cause over one million infections, 19000 hospitalizations and over 400 deaths annually in the US (Forkus et al, 2017), with some Salmonella serovars in food showing antibiotic resistance. Although salmonellosis incidents are traditionally relatively low in Australia, recent egg-associated outbreaks have brought this back to the attention of the regulators and consumers.
It is now possible to cause a targeted bacterium to self-destruct through the use of CRISPR, the biological sequences that make up the bacterial immune system (Hamilton et al., 2019). This technology is extremely precise, such that it can target a specific bacterium or a defined range of bacteria. This means that, unlike many antibiotics, it can be used to remove only the unwanted bacteria in the animal gut microbiome and leave beneficial gut flora unchanged, potentially enhancing the well-being of the animal. One way to induce bacteria in the animal gut to self-destruct is to introduce a suitable plasmid into the target organism(s) through conjugation via a probiotic included in the feed or drinking water. The current trial looks at the ability of this technology, named Guided Biotics™, to reduce Salmonella colonization in challenged broilers.
II. METHOD
A non-pathogenic Escherichia coli strain was used as the vector in this trial, and was loaded with a plasmid including a CAS sequence and 3 target sequences specific to all Salmonella serovars (Guided Biotics™). Ross 308 as-hatched birds (165) were obtained on day of hatch and housed under controlled biosecure conditions, with access to water and standard commercial rations ad libitum. Birds were dosed continually from day 1 with either:
1. No addition to water (45 birds)
2. Unmodified E. coli vector at 108 cfu/ml drinking water (45 birds)
3. Anti-Salmonella Guided BioticsTM at 108 cfu/ml drinking water (45 birds)
In parallel, a group of 30 birds was dosed orally with 0.5 ml 105 CFU/mL Salmonella Enteritidis strain FS26 on day 1. Birds were checked for Salmonella colonisation at day 3 by cloacal swab (ISO 6579-1:2017). On day 5, three verified Salmonella-colonised birds (seeder birds, with > 105 CFU/g in swabs) were marked and added to each of the test groups.
Fifteen non-seeder birds from each group were euthanased on day 12 (7 days post-mixing with seeder birds) and caecal contents were counted for Salmonella using both direct and enhanced methods. Caecal samples were serially diluted in PBS before plating onto XLD agar for direct counts, whilst for enhanced counts the samples were first incubated in Selenite Cystine broth for 18 hrs at 41°C before plating and counting (ISO 6579-1:2017). For the purpose of data transformation, samples negative in either method were allocated a count of 1 CFU/g, while those negative in direct counts but positive in the enhanced method were allocated 500 CFU/g. Body weights of the remaining birds were monitored at day 42. Counts and weights were log transformed and statistical analysis conducted using GraphPad Prism. Data were assessed for normality of distribution using a D’Agostino and Pearson omnibus normality test and non-normal were analysed using a Kruskall-Wallis test with Dunn’s multiple comparison test post hoc. Differences were analysed using Fisher’s exact test.
III. RESULTS
All birds in the seeder group showed cloacal Salmonella counts of > 105 CFU/g by day 3. By day 12 (7 days post introduction of seeder birds to test groups) all birds in the Control and E. coli vector-only groups were positive using the enhanced counts method, exhibiting caecal counts of 500-4,000,000 CFU/g (Table 1). Twenty two of these 30 birds were also positive with direct counts. However, when the anti-Salmonella Guided BioticsTM was added to the drinking water, Salmonella was not detected in any birds with the direct method, and only 8 of the 15 birds tested were positive with enhanced counts. The Guided BioticsTM treatment reduced (P < 0.001) mean Salmonella counts by approximately log-3 (from log 4.12 to log 1.26, equivalent to 14,200 CFU/g to 18 CFU/g) and also improved 42-day liveweight by 15% (P = 0.02; Figure 1).
Table 1 - Influence of the E. coli vector alone or Guided Biotics™ with an anti-Salmonella plasmid on caecal Salmonella counts (log10 CFU/g, enhanced counts method) in 12-day old Salmonella-challenged broilers.
Figure 1 - Influence of Guided Biotics™ on bird liveweight at 42 days of age (g).
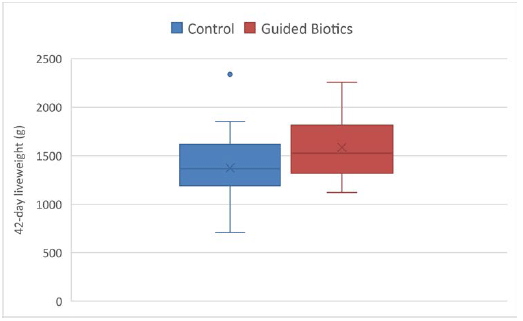
The challenge method employed in this study is consistent with that often use in Salmonella vaccine tests and may be regarded as severe (Cooper et al., 1994). All seeder birds were infected when introduced into the test pens, and the Salmonella shed to in-contact birds would be expected to be highly infective. This was confirmed by the universally high caecal counts in all Control birds 7 days after seeded-bird introduction. Conversely, the Guided BioticsTM, delivered by conjugation in the digestive tract, was able to stop Salmonella colonization in 8 out of 15 (53%) of the test birds. The average Salmonella count in caecal digesta was also reduced by approximately log-3 (thousand-fold) and the maximum Salmonella count lowered from 4 million CFU/g in Control birds to 500 CFU/g in Guided BioticsTM treated. The 15% increase in liveweight of birds fed the Guided BioticsTM relative to the Control birds further indicates the severity of the Salmonella challenge employed in this trial. The lack of any effect of the E. coli vector on colonization confirms that the Guided BioticsTM plasmid was essential for Salmonella reduction.
This initial trial establishes the capability of Guided Biotics™ technology to specifically remove unwanted bacteria, in this case a single Salmonella serovar. The tested Guided BioticTM is designed to target all known 2400 Salmonella serovars, and laboratory trials have established efficacy across the main serovars involved in human food poisoning. Ongoing laboratory tests have also indicated that solutions for other unwanted bacteria, such as Clostridium perfringens and Avian Pathogenic E. coli, are feasible. Furthermore, because the design of the targeting is specific, tests have confirmed that off-target killing of desirable or commensal bacteria can be avoided. It is clear that this Guided Biotics™ technology has the potential to make a substantial contribution to the replacement of antibiotics in poultry production, reduce zoonosis incidents and maintain bird performance in antibiotic-free diets.
Presented at the 31th Annual Australian Poultry Science Symposium 2020. For information on the next edition, click here. 











.jpg&w=3840&q=75)