The impact of porcine spray-dried plasma protein and dried egg protein harvested from hyper-immunized hens, provided in the presence or absence of subtherapeutic levels of antibiotics in the feed, on growth and indicators of intestinal function and physiology of nursery pigs
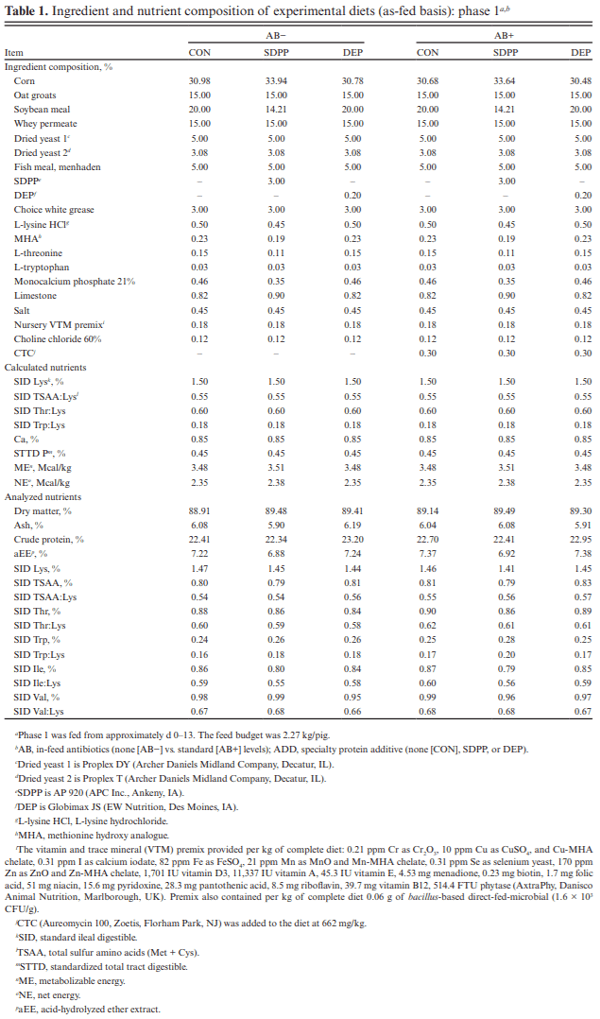
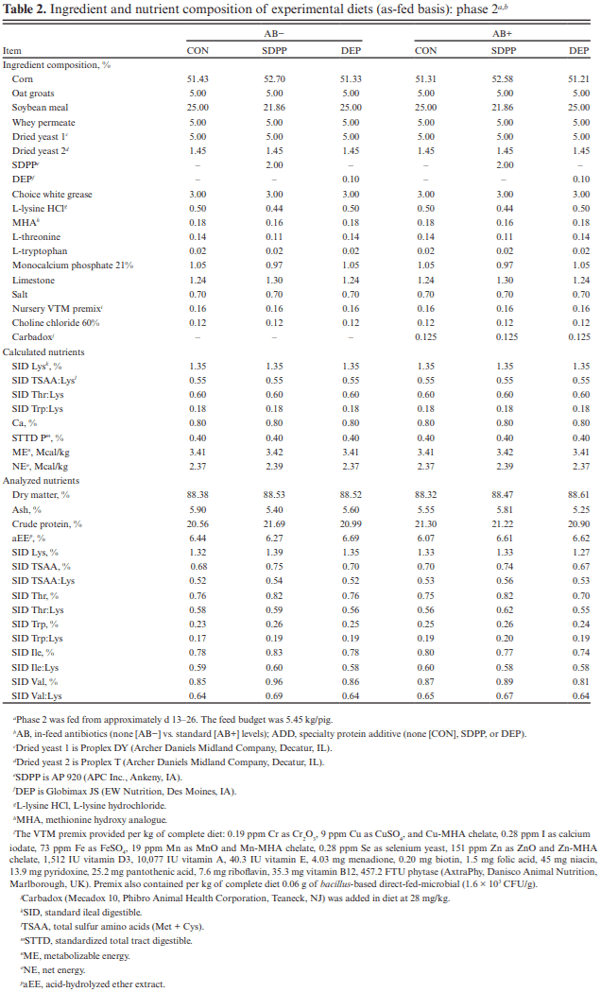
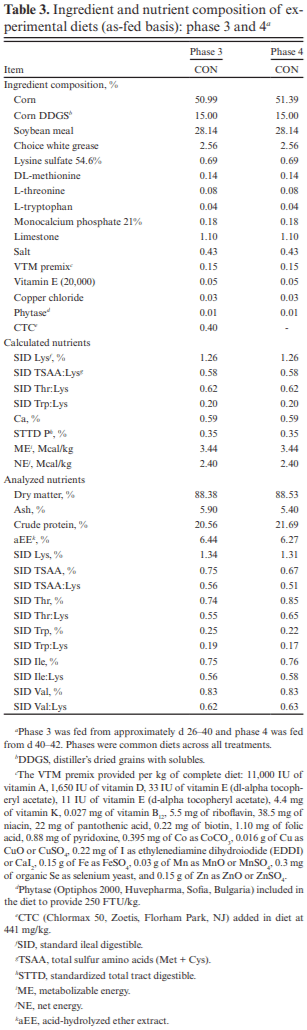
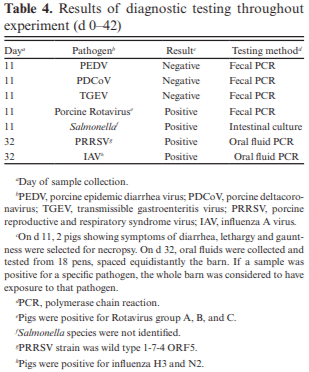
The objective of this experiment was to compare the effects of spray-dried plasma protein (SDPP) and dried egg protein (DEP), without (AB−) or with (AB+) in-feed antibiotics, on growth performance and markers of intestinal health in nursery pigs raised in commercial conditions. This 42-d experiment utilized 1,230 pigs (4.93 ± 0.04 kg body weight; approximately 15–18 d of age). Pigs were randomly assigned to one of six dietary treatments that were arranged as a 2 × 3 factorial of in-feed antibiotics (AB− vs. AB+) and a specialty protein additive (none [CON], porcine SDPP, or DEP). Diets were fed in four phases with phases 3 and 4 as a common diet across all treatments. Specialty protein additives were fed in phases 1 (0–13 d; 3% SDPP, and 0.20% DEP) and 2 (13–26 d; 2% SDPP, and 0.10% DEP). Antibiotics were fed in phases 1–3 (662 mg chlortetracycline [CTC]/ kg, 28 mg carbadox/kg, and 441 mg CTC/kg, respectively). Ileal tissue and blood samples were collected from 48 pigs (8 per treatment) on d 20. Data were analyzed using PROC MIXED of SAS (9.4) with pen as the experimental unit; protein additives, antibiotics, and their interaction were fixed effects and block was a random effect. The pigs experienced naturally occurring health challenges in weeks 2 and 4. In the AB− diets, SDPP and DEP increased average daily gain (ADG; P = 0.036) and average daily feed intake (ADFI; P = 0.040) compared to CON; in the AB+ diets, neither SDPP nor DEP increased ADG or ADFI compared to CON but SDPP did increase these parameters over DEP. The SDPP and DEP diets decreased the number of individual medical treatments compared to CON (P = 0.001). The AB+ increased ileal mucosal interleukin (IL)-1 receptor antagonist (P = 0.017). Feeding DEP reduced the concentration of mucosal IL-1β compared to CON, but not SDPP (P = 0.022). There was a trend for SDPP and DEP to increase villus height:crypt depth compared to CON (P = 0.066). Neither antibiotics or protein additive affected serum malondialdehyde concentration or ileal mRNA abundance of claudin-3 or 4, occludin, or zonula occludens-1 (P > 0.10). In conclusion, SDPP and DEP improved growth performance of weaned pigs in the absence of anti-biotics but neither improved growth compared to CON when feeding standard antibiotic levels. The specialty proteins had a positive effect on health; specialty proteins and antibiotics were able to modulate some markers of intestinal inflammation and morphology.
Key words: functional protein, IgY, in-feed antibiotics, intestinal inflammation and morph-ology, spray-dried plasma protein, weaned pig.
Abbas, A. T., S. A. El-Kafrawy, S. S. Sohrab, and E. I. A. Azhar. 2019. IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum. Vaccin. Immunother. 15:264–275. doi:10.1080/21645515.2018.15 14224
Al-Sadi, R., M. Boivin, and T. Ma. 2009. Mechanism of cyto-kine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed). 14:2765–2778. doi:10.2741/3413
AOAC. 2007. Official methods of analysis of AOAC International. 18th ed. Gaithersburg (MD): AOAC International.
Armstrong, D., and R. Browne. 1994. The analysis of free radicals, lipid peroxides, antioxidant enzymes and com-pounds related to oxidative stress as applied to the clin-ical chemistry laboratory. In: D. Armstrong, editor, Free radicals in diagnostic medicine. Boston (MA): Springer; p. 43–58.
Awad, W. A., C. Hess, and M. Hess. 2017. Enteric pathogens and their toxin-induced disruption of the intestinal bar-rier through alteration of tight junctions in chickens. Toxins. 9:60. doi:10.3390/toxins9020060
Bikker, P., A. J. van Dijk, A. Dirkzwager, J. Fledderus, M. Ubbink-Blanksma, and A. C. Beynen. 2004. The in-fluence of diet composition and an anti-microbial growth promoter on the growth response of weaned piglets to spray dried animal plasma. Livest. Prod. Sci. 86:201–208. doi:10.1016/j.livprodsci.2003.07.003
Bosi, P., L. Casini, A. Finamore, C. Cremokolini, G. Merialdi, P. Trevisi, F. Nobili, and E. Mengheri. 2004. Spray-dried plasma improves growth performance and reduces inflam-matory status of weaned pigs challenged with enterotoxi-genic Escherichia coli K88. J. Anim. Sci. 82:1764–1772. doi:10.2527/2004.8261764x
Coffey, R. D., and G. L. Cromwell. 1995. The impact of envir-onment and antimicrobial agents on the growth response of early-weaned pigs to spray-dried porcine plasma. J. Anim. Sci. 73:2532–2539. doi:10.2527/1995.7392532x
Corl, B. A., R. J. Harrell, H. K. Moon, O. Phillips, E. M. Weaver, J. M. Campbell, J. D. Arthington, and J. Odle. 2007. Effect of animal plasma proteins on intes-tinal damage and recovery of neonatal pigs infected with rotavirus. J. Nutr. Biochem. 18:778–784. doi:10.1016/j. jnutbio.2006.12.011
Crenshaw, J. D., J. M. Campbell, J. Polo, and H. H. Stein. 2017. Effects of specialty proteins as alternatives to bovine or porcine spray-dried plasma in non-medicated diets fed to weaned pigs housed in an unsanitary environment. Transl. Anim. Sci. 1:333–342. doi:10.2527/tas2017.0040
Cromwell, G. L. 2002. Why and how antibiotics are used in swine production. Anim. Biotechnol. 13:7–27. doi:10.1081/ABIO-120005767
Ermer, P. M., P. S. Miller, and A. J. Lewis. 1994. Diet preference and meal patterns of weanling pigs offered diets containing either spray-dried porcine plasma or dried skim milk. J. Anim. Sci. 72:1548–1554. doi:10.2527/1994.7261548x
FASS. 2010. Guide for the care and use of agricultural ani-mals in research and teaching, 3rd ed. Champaign (IL): Federation of Animal Science Societies.
Foss, D. L., M. J. Zilliox, and M. P. Murtaugh. 2001. Bacterially induced activation of interleukin-18 in porcine intestinal mucosa. Vet. Immunol. Immunopathol. 78:263–277. doi:10.1016/s0165-2427(00)00266-x
France, M. M., and J. R. Turner. 2017. The mucosal barrier at a glance. J. Cell Sci. 130:307–314. doi:10.1242/jcs.193482
Gao, Y., F. Han, X. Huang, Y. Rong, H. Yi, and Y. Wang. 2013. Changes in gut microbial populations, intestinal morph-ology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J. Anim. Sci. 91:5614–5625. doi:10.2527/jas.2013-6528
Gaskins, H. R., C. T. Collier, and D. B. Anderson. 2006. Antibiotics as growth promotants: mode of action. Anim. Biotechnol. 13:29–42. doi:10.1081/ABIO-12000576
Gerber, P. F., C. Xiao, Q. Chen, J. Zhang, P. G. Halbur, and T. Opriessnig. 2014. The spray-drying process is suffi-cient to inactivate infectious porcine epidemic diarrhea virus in plasma. Vet. Microbiol. 174:86–92. doi:10.1016/j. vetmic.2014.09.008
Heo, J. M., T. A. Woyengo, R. K. Kahindi, E. Kiarie, P. K. Maiti, and C. M. Nyachoti. 2015. Ileal amino acid digestibility in egg from hyperimmunized-hens fed to weaned pigs and piglet response to diets contain egg products. Anim. Feed Sci. Tech. 204:52–61. doi:10.1016/j.anifeedsci.2015.03.006
Hu, C. H., K. Xiao, Z. S. Luan, and J. Song. 2013. Early weaning increases intestinal permeability, alters expres-sion of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi:10.2527/jas.2012-5796
Huntley, N. F., C. M. Nyachoti, and J. F. Patience. 2018. Lipopolysaccharide immune stimulation but not β-man-nanase supplementation affects maintenance energy re-quirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi:10.1186/s40104-018-0264-y
Jacela, J. Y., J. M. DeRouchey, M. D. Tokach, R. D. Goodband, J. L. Nelssen, D. G. Renter, and S. S. Dritz. 2009. Feed addi-tives for swine: fact sheets-acidifiers and antibiotics. J. Swine Health Prod. 17:270–275. doi:10.4148/2378–5977.7071
Lallès, J. P., G. Boudry, C. Favier, N. Le Floc’h, I. Luron, L. Montagne, I. P. Oswald, S. Pié, C. Piel, and B. Sève. 2004. Gut function and dysfunction in young pigs: physiology. Anim. Res. 53:301–316. doi:10.1051/animres:2004018
Lallès, J. P., P. Bosi, H. Smidt, and C. R. Stokes. 2007. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66:260–268. doi:10.1017/ S0029665107005484
Li, Q., E. R. Burrough, N. K. Gabler, C. L. Loving, O. Sahin, S. A. Gould, and J. F. Patience. 2019. A soluble and highly fermentable dietary fiber with carbohydrases improved gut barrier integrity markers and growth performance in F18 ETEC challenged pigs1. J. Anim. Sci. 97:2139–2153. doi:10.1093/jas/skz093
Li, X., L. Wang, Y. Zhen, S. Li, and Y. Xu. 2015. Chicken egg yolk antibodies (IgY) as non-antibiotic production en-hancers for use in swine production: a review. J. Anim. Sci. Biotechno. 6:40. doi:10.1186/s40104-015-0038-8
Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402– 408. doi:10.1006/meth.2001.1262
Mankertz, J., S. Tavalali, H. Schmitz, A. Mankertz, E. O. Riecken, M. Fromm, and J.D. Schulzke. 2000. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J. Cell Sci. 113 (Pt 11):2085–2090.
Montagne, L., G. Boudry, C. Favier, I. Le Huërou-Luron, J. P. Lallès, and B. Sève. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 97:45–57. doi:10.1017/S000711450720580X
Nabuurs, M. J., A. Hoogendoorn, E. J. van der Molen, and A. L. van Osta. 1993. Villus height and crypt depth in weaned and unweaned pigs, reared under various cir-cumstances in The Netherlands. Res. Vet. Sci. 55:78–84. doi:10.1016/0034-5288(93)90038-h
Netea, M. G., F. L. van de Veerdonk, J. W. van der Meer, C. A. Dinarello, and L. A. Joosten. 2015. Inflammasome-independent regulation of IL-1-family cyto-kines. Annu. Rev. Immunol. 33:49–77. doi:10.1146/ annurev-immunol-032414-112306
Nofrarías, M., E. G. Manzanilla, J. Pujols, X. Gibert, N. Majó, J. Segalés, and J. Gasa. 2006. Effects of spray-dried por-cine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J. Anim. Sci. 84:2735–2742. doi:10.2527/jas.2005-414
NRC. 2012. Nutrient requirements of swine, 11th ed. Washington (DC): The National Academies Press.
Olsen, K. M., N. K. Gabler, C. J. Rademacher, K. J. Schwartz, W. P. Schweer, G. G. Gourley, and J. F. Patience. 2018. The effects of group size and subtherapeutic antibiotic alter-natives on growth performance and morbidity of nursery pigs: a model for feed additive evaluation. Transl. Anim. Sci. 2:298–310. doi:10.1093/tas/txy068
Owusu-Asiedu, A., C. M. Nyachoti, S. K. Baidoo, R. R. Marquardt, and X. Yang. 2003. Response of early- weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody. J. Anim. Sci. 81:1781–1789. doi:10.2527/2003.8171781x
Owusu-Asiedu, A., C. M. Nyachoti, and R. R. Marquardt. 2003. Response of early-weaned pigs to an entero-toxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fu-maric acid, or antibiotic. J. Anim. Sci. 81:1790–1798. doi:10.2527/2003.8171790x
Patience, J. F. 2019. Feeding and management for antibiot-ic-reduced and antibiotic-free pork production. AFMA Matrix. 28:23–29. doi:https://hdl.handle.net/10520/ EJC-1786d9f9eb
Patterson, A. R., D. M. Madson, and T. Opriessnig. 2010. Efficacy of experimentally produced spray-dried plasma on infectivity of porcine circovirus type 2. J. Anim. Sci. 88:4078–4085. doi:10.2527/jas.2009-2696
Peace, R. M., J. Campbell, J. Polo, J. Crenshaw, L. Russell, and A. Moeser. 2011. Spray-dried porcine plasma influ-ences intestinal barrier function, inflammation, and diar-rhea in weaned pigs. J. Nutr. 141:1312–1317. doi:10.3945/ jn.110.136796
Pérez-Bosque, A., J. Polo, and D. Torrallardona. 2016. Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porcine Health Manag. 2:16. doi:10.1186/s40813-016-0034-1
Pettigrew, J. E. 2006. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: dietary tools, part 1. Anim. Biotechnol. 17:207–215. doi:10.1080/10495390600956946
Pié, S., J. P. Lallès, F. Blazy, J. Laffitte, B. Sève, and I. P. Oswald. 2004. Weaning is associated with an upregulation of ex-pression of inflammatory cytokines in the intestine of pig-lets. J. Nutr. 134:641–647. doi:10.1093/jn/134.3.641
Pierce, J. L., G. L. Cromwell, M. D. Lindemann, L. E. Russell, and E. M. Weaver. 2005. Effects of spray-dried animal plasma and immunoglobulins on perform-ance of early weaned pigs. J. Anim. Sci. 83:2876–2885. doi:10.2527/2005.83122876x
Pluske, J. R. 2013. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 4:1. doi:10.1186/2049-1891-4-1
Pluske, J. R., D. J. Hampson, and I. H. Williams. 1997. Factors influencing the structure and function of the small intes-tine in the weaned pig: a review. Livest. Prod. Sci. 51:215– 236. doi:10.1016/S0301-6226(97)00057-2
Pozzebon da Rosa, D., M. de Moraes Vieira, A. M. Kessler, T. Martin de Moura, A. P. G. Frazzon, C. M. McManus, F. R. Marx, R. Melchior, and A. M. L. Ribeiro. 2015. Efficacy of hyperimmunized hen egg yolks in the control of diarrhea in newly weaned piglets. Food Agr. Immunol. 26:622–634. doi:10.1080/09540105.2014.998639
Prickett, J. R., W. Kim, R. Simer, K. J. Yoon, and J. Zimmerman. 2008. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J. Swine Health Prod. 16:86–91.
Schade, R., E. G. Calzado, R. Sarmiento, P. A. Chacana, J. Porankiewicz-Asplund, and H. R. Terzolo. 2005. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 33:129–154. doi:10.1177/026119290503300208
Torrallardona, D., E. Esteve-García, and J. Brufau. 2002. Use of spray dried animal plasma as an alternative to anti-microbial medication in weanling pigs. Anim. Feed Sci. Technol. 99:119–129. doi:10.1016/S0377-8401(02)00072-X Torrallardona, D., and J. Polo. 2016. Effect of spray-dried por-cine plasma protein and egg antibodies in diets for weaned pigs under environmental challenge conditions. J. Swine Health Prod. 24:21–28.
Tran, H., J. W. Bundy, Y. S. Li, E. E. Carney-Hinkle, P. S. Miller, and T. E. Burkey. 2014. Effects of spray-dried porcine plasma on growth performance, immune response, total antioxidant capacity, and gut morph-ology of nursery pigs. J. Anim. Sci. 92:4494–4504. doi:10.2527/jas.2014–7620
Turner, J. L., S. S. Dritz, J. J. Higgins, K. L. Herkelman, and J. E. Minton. 2002. Effects of a Quillaja saponaria extract on growth performance and immune function of weanling pigs challenged with Salmonella typhimurium. J. Anim. Sci. 80:1939–1946. doi:10.2527/2002.8071939x
van Dijk, A. J., H. Everts, M. J. A. Nabuurs, R. J. C. F. Margry, and A. C. Beynen. 2001. Growth performance of weanling pigs fed spray-dried animal plasma: a re-view. Livest. Prod. Sci. 68:263–274. doi:10.1016/ S0301-6226(00)00229-3
Wang, Z., J. Li, J. Li, Y. Li, L. Wang, Q. Wang, L. Fang, X. Ding, P. Huang, J. Yin, Y. Yin, and H. Yang. 2019. Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhe-sion in weaned piglets. BMC Vet. Res. 15:234. doi:10.1186/ s12917-019-1958-x
Wiedemann, V., E. Linckh, R. Kühlmann, P. Schmidt, and U. Lösch. 1991. Chicken egg antibodies for prophylaxis and therapy of infectious intestinal diseases. J. Vet. Med. B 38:283–291. doi:10.1111/j.1439-0450.1991. tb00872.x
Yagi, K. 1998. Simple assay for the level of total lipid peroxides in serum or plasma. In: D. Armstrong, editor, Free radical and antioxidant protocols. Totowa (NJ): Humana Press; p. 101–106.
Youakim, A., and M. Ahdieh. 1999. Interferon-gamma decreases barrier function in T84 cells by re-ducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. 276:G1279–G1288. doi:10.1152/ ajpgi.1999.276.5.G1279
Zhang, Y., P. Zheng, B. Yu, J. He, J. Yu, X. B. Mao, J. X. Wang, J. Q. Luo, Z. Q. Huang, G. X. Cheng, et al. 2016. Dietary spray-dried chicken plasma improves intestinal barrier function and modulates immune status in weaning piglets. J. Anim. Sci. 94:173–184. doi:10.2527/jas.2015-9530











.jpg&w=3840&q=75)