Introduction
All over the world, E. coli is an important cause of a wide range of diseases in pigs, including postweaning diarrhea (PWD) (Fairbrother et al., 2019). Diarrhea due to E. coli may result in significant economic losses due to morbidity, mortality, decreased weight gain, and cost of treatment, vaccinations and feed supplements. Clinical signs fluctuate with time and regions and may range from mild diarrhea with 1.5 to 2% mortality and lower weight gain to severe diarrhea or sudden death with up to 25% mortality. PWD may be endemic or occur as outbreaks, and most commonly occurs in the first days through to several weeks after weaning and even after introduction into fattening herds. E. coli is the most common cause of PWD, although Salmonella and rotavirus may be associated with this disease and mixed infections may occur. Risk factors associated with the pigs (i.e. stress or level of immunity), and environment (biosecurity management on the farm) have an important influence on the occurrence of disease.
E. coli is a Gram-negative bacterial rod that inhabits the intestinal microflora or ecosystem of most mammalian and bird species, including the pig. Most E. coli are commensals, that is, they reside in the intestine but are not harmful for the host animal. Only a small proportion of isolates are pathogenic, being classified into categories or pathotypes based on the production of broad classes of virulence factors and on the mechanisms by which they cause disease.
Strains of the most important pathotype in pigs, the enterotoxigenic E. coli (ETEC), produce one or several of a class of toxins called enterotoxins, which act on the intestinal epithelial cells to induce the secretion of water and electrolytes into the intestinal lumen, causing the clinical signs of diarrhea. The most important enterotoxins, which define ETEC, are the heat labile toxin LT and the heat stable toxins STa and STb. ETEC must be able to adhere to and colonise the intestinal mucosa to permit the release of sufficient levels of enterotoxin to result in the development of diarrhea. This adherence is mediated by hair-like structures on the bacterial surface, called fimbriae or pili. ETEC associated with neonatal diarrhea may produce one or more of the fimbriae F4, F5, F6, and F41. The first three fimbriae are also known as K88, K99, and 987P. ETEC associated with postweaning diarrhea most commonly produce F4(K88) or F18 fimbriae.
A second pathotype found in pigs with diarrhea is known as enteropathogenic E. coli (EPEC). EPEC were initially associated with diarrhea in children, especially in developing countries. These bacteria cause typical attaching and effacing lesions, and possess a variant of the EPEC attaching effacing factor Eae or Intimin.
Shiga toxin producing E. coli (STEC) produce one or more of a family of cytotoxins which are known collectively as Shiga toxins (Stx) or verotoxins (VT). In pigs, the most important STEC are those that cause edema disease. These strains produce the toxin variant Stx2e (VT2e) and the fimbriae F18. Certain isolates produce both Stx2e and enterotoxins, as well as the fimbriaeF18. These isolates are associated more with PWD than edema disease. Such isolates are designated as ETEC/STEC hybrids.
More complete characterisation of isolates is performed in reference laboratories by serotyping, and phylogenetic analysis using such techniques as multi-locus sequence typing (MLST),pulse-field gel electrophoresis (PFGE), and more recently by whole genome sequencing. This type of characterisation will allow the monitoring of changing trends and the identification of new, emerging E. coli virulence determinants that could gain importance due to the pressure of antimicrobial therapy.
E. coli bacteria are constantly shed into the immediate environment of the pig via the feces, and contaminate the pens, farrowing crates, and floor. They can persist for long periods, possibly more than 10 weeks. E. coli is transmitted to pigs and other animals via contaminated feed, handlers, and drinking water, and infection occurs by the oral route. E. coli from pigs may also be transmitted to humans by direct contact, or ingestion of food or water contaminated following spread of manure, or ingestion of meat following contamination of carcasses at the slaughterhouse. Intestinal infections due to ETEC and edema disease STEC are often considered to be contagious, the same strain being found in several sick pigs from one pen and from one batch to another. These strains are usually only shed for a few days after infection, probably due to the development of immunity, and might not be detected at the time of appearance of clinical signs.
This paper aims to address how the pathogenic E. coli profile is changing in the face of recent pressures such as the overuse of antimicrobials, more intensive animal management and greater movement of animals, and the use of more sophisticated techniques for the identification and monitoring of pathogenic E. coli.
Detection of pathogenic E. coli associated with postweaning diarrhea
Rapid detection and thorough identification of pathogenic E. coli permit an early, accurate diagnosis of disease caused by these bacteria. This allows a timely and judicious choice of antimicrobial agent for effective treatment of affected animals and control of an outbreak. Accurate diagnosis also permits an informed decision for putting into place the most appropriate and efficacious preventive and control strategies, such as vaccination and management changes.
Currently, genotypic analysis such as polymerase chain reaction (PCR) is most commonly used to define the virotypes involved in an infection (Luppi, 2017). This test permits the detection of genes encoding for virulence factors such as toxins and adhesins. Primers recognising different genes related to toxins (STa, STb, and LT) and adhesins (F4, F5, F6, F18, F41, AIDA) for ETEC strains; attachment and effacement, such as eae; and Stx for STEC strains are readily available and can be used to perform PCR.
As E. coli grows rapidly and easily in routine culture conditions, a simple, sensitive, and inexpensive approach for the detection of the presence of pathogenic E. coli in samples is to perform PCR directly on DNA prepared from bacteria grown in an enrichment broth culture medium. This approach is used routinely at the EcL (Rhouma et al., 2017). For instance, multiplex PCR amplification may be used to detect the genes encoding for the enterotoxins of ETEC, Shiga toxins of STEC, and Eaeof EPEC, associated with diarrhea or edema disease. This approach is rapid and indicates overnight the presence of pathogenic E. coli, identifying the pathotype(s) involved in a particular case. However, it does not permit the identification of specific virotypes, as is possible when colonies are tested. Isolates from pathotype-positive cases may then be virotyped by PCR. Identification of the causative agent of disease will permit a more appropriate choice of isolate(s) for antimicrobial resistance testing. In many laboratories, hemolytic isolates are selected for further virotyping.
In a particular case, ETEC colonies positive for one of the adhesins F4, F5, F6, F41, or F18, or STEC positive for F18, when present, could be considered the causative agent of the presenting diarrhea or edema disease, as these virotypes are rarely present in the intestinal microflora of normal pigs. On the other hand, EPEC or ETEC positive for AIDA, when present, are considered as opportunistic agents of diarrhea, as strains of these virotypes have been demonstrated to cause diarrhea in pigs in challenge experiments but are also present in the intestinal microflora of normal pigs. Similarly, ETEC or STEC possessing no known adhesins, when present, would be considered as possible agents of diarrhea or edema disease, as strains of these virotypes have not yet been demonstrated to cause disease in pigs, and are also found in the intestinal microbiota of normal pigs.
Recent data on the virulence gene profile of pathogenic E. coli associated with postweaning diarrhea in various countries
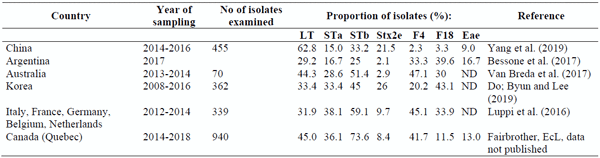
An overview of recent published data(Table 1); Luppi et al. (2016); Yang et al. (2019); Bessone et al. (2017); Van Breda et al. (2017); Do et al. (2019) and of data reported from our laboratory (EcL)on the presence of virulence genes in E. coli isolated from cases of postweaning diarrhea suggests that there are some differences between countries in the prevalence of the different pathotypes. Care should be taken in interpretation of these results due to differences between studies in the methods for detection of positive isolates.Also, the combinations of virulence genes found in individual isolates were not always reported for each study.
The proportion of Stx2e-positive isolates was higher in certain countries, such as China and Korea, suggesting a higher prevalence of STEC in those countries (Table 1). Also, the data show that the relative importance of F4-positive and F18-positive isolates varies greatly from one country to another.In addition, Luppi et al. (2016) found a variation in relative importance of F4-positive and F18-positive isolates between the European countries in their study. In contrast to the situation in most other countries, F4-positive isolates are far more prevalent than F18-positive isolates in Québec, Canada.
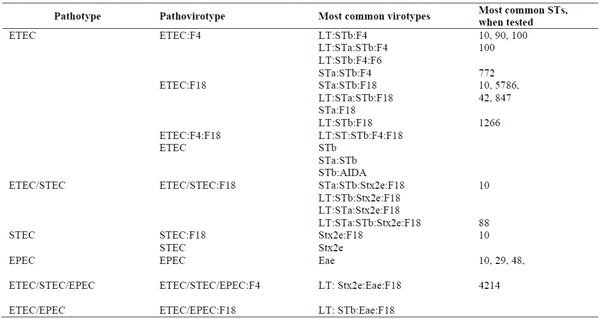
Table 2 summarizes the pathovirotypes and virotypes most commonly observed, when reported, in the above and other recent studies for which the virulence gene combinations of isolates were reported Garcia-Menino et al. (2018); Mohlatlole et al. (2013); Brand et al. (2017). At the EcL, we refer to the combination of pathotype and fimbrial adhesin as the «pathovirotype», and use this designation in reporting results to veterinarians. This provides relevant information regarding the choice of prevention measures that may be used, e.g. a vaccine specific for a fimbrial adhesion such as F4 or F18, without the complication of details on enterotoxins, which are not necessary for such decisions.
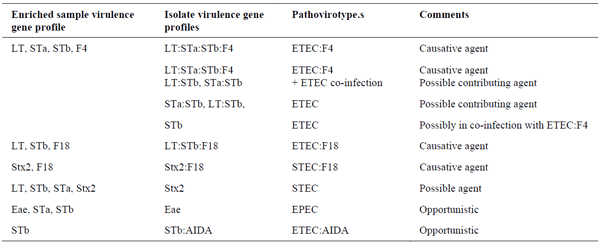
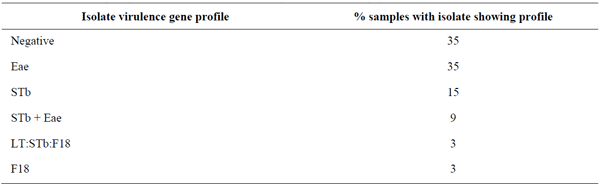
The prevalence of F4- and F18-positive isolates in the Chinese study was very low(Yang et al., 2019) whereas the prevalence of LT-positive isolates was much higher, suggesting a high prevalence of ETEC negative for both F4 and F18 (Table 1). Similarly, we observed a higher prevalence of LT-positive isolates at the EcL. In addition, we often observe samples in which ETEC isolates negative for F4 and F18 are found alone, or in combination with ETEC:F4 or ETEC:F18 positive isolates (Table 3). Enriched primary cultures from these samples from pigs with PWD are often positive for one or more of the enterotoxins but negative for F4 and F18. In contrast, both enriched cultures and isolates from samples from healthy pigs on 12 different farms (Table 4) demonstrated a very low prevalence of virulence genes.
These results suggest the presence of previously unknown pathogenic isolates or secondary pathogens/opportunists that contribute to the development of diarrhea in certain circumstances such as co-infections and that should be taken into consideration when looking at prevention measures.
Table 1. Prevalence of virulence genes in pathogenic E. coli from pigs with postweaning diarrhea in different countries.
Table 2. Pathotypes, pathovirotypes, and virotypes of E. coli isolates commonly observed in postweaning diarrhea in pigs.
Table 3. Common virulence gene profiles in clinical cases with intestinal problems in pigs at EcL.
Table 4. Virulence gene profiles in isolates from 34 samples from healthy pigs on 12 different farms
Emergence of clonal clusters of antimicrobial resistant pathogenic E. coli in postweaning pigs
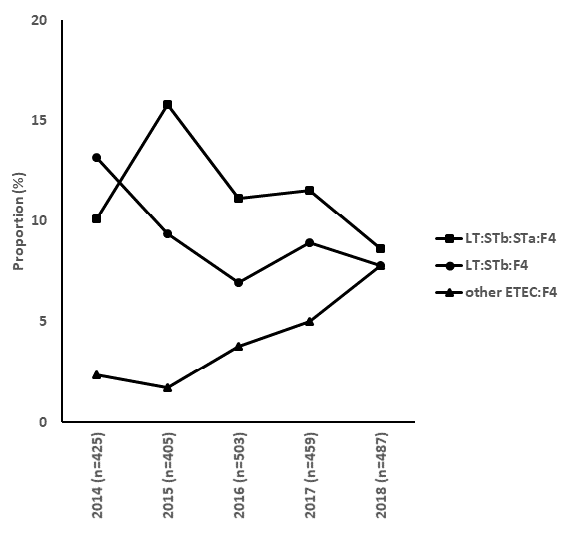
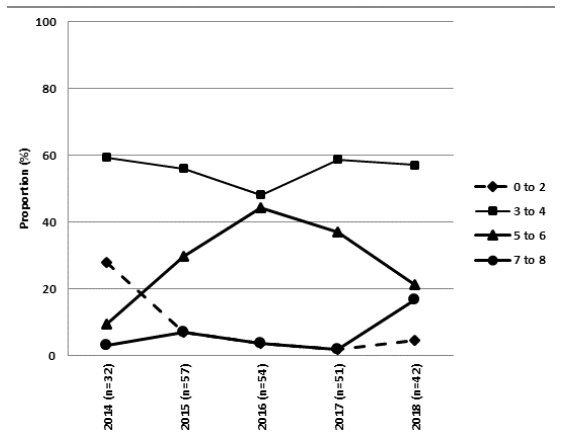
In 2014, a new ETEC:F4 virotype, LT:STb:STa:F4, appeared and became predominant in cases of diarrhea in pigs in Quebec province (Figure 1) (Fairbrother, data not published). Up to 2014, the predominant virotype was LT:STb:F4. Isolates were nonsusceptible to enrofloxacin and have been becoming increasingly multidrug resistant (Figure 2). PFGE analysis revealed the presence of a clonal cluster that emerged in 2015 and has subsequently spread throughout the province of Quebec. The prevalence of cases associated with this cluster appears to have decreased more recently. From 2016, another new ETEC:F4 virotype, STb:STa:F4, has appeared and continues to become more prevalent (Figure 2, «other ETEC:F4»). We will continue to monitor isolates of this virotype in order to determine if a new multidrug resistant pathogenic clonal cluster is emerging.
Similarly, Kusumoto et al. (2016) described the emergence in 2003-2005 of an ETEC/STEC clonal cluster based on PFGE analysis. Isolates belonged to ST88 and to virotypes LT:STa:STb:Stx2e:F18, were predominantly fluoroquinolone-resistant, and demonstrated a high level of MDR.
Figure 1. Proportion of cases positive for the various ETEC:F4 virotypes in Quebec, Canada, by year.
Figure 2. Multidrug resistance of LT:STb:STa:F4 isolates from diseased pigs in Quebec, Canada, by year. Isolates were tested for resistance to 8 classes of antimicrobials commonly used for treatment of bacterial infections in pigs.
Prevalent pig E. coliclonal lineages may have adaptive advantages due to antimicrobial resistance
As shown in Table 2, many pig pathogenic E. coli of the various pathotypes belong to the clonal lineage ST10. Brilhante et al. (2019), using whole genome sequencing, recently showed that the ETEC and STEC virulence genes of a hybrid ETEC/STEC:F18 ST10 strain were carried on a single plasmid, suggesting a key role for plasmids in the emergence of hybrid pathotypes. Multiple resistance genes were carried on two conjugative plasmids on this same strain. Garcia-Menino et al. (2019), also using whole genome sequencing, identified hybrid ETEC/STEC:F18, ETEC:F18, and ETEC:F4 strains of clonal lineage ST10 that possessed chromosomal mutations both for fluoroquinolone and colistin resistance, as well as plasmid-located mcr genes conferring colistin resistance and resistance genes for other antimicrobials.
Conclusions
It appears that persistent, multidrug resistant, pathogenic E. coli clones associated with postweaning diarrhea in pigs are now emerging more frequently. The additive effect of the presence of the different antimicrobial resistance genes or mutations, often both chromosomal and plasmid-located, could be conferring adaptive advantages to clonal lineages such as ST10 and driving an increased virulence andability to emerge, spread, and persist, and allowing these lineages to predominate. Tools such as whole genome sequencing and associated databases such as that for MLST will facilitate the worldwide monitoring of the emergence of new clonal lineages.
Published in the proceedings of the International Pig Veterinary Society Congress – IPVS2020. For information on the event, past and future editions, check out https://ipvs2022.com/en.