Seasonal variation in sperm characteristics of boars in southern Uruguay
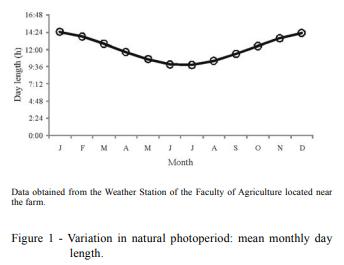
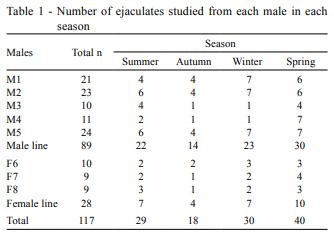
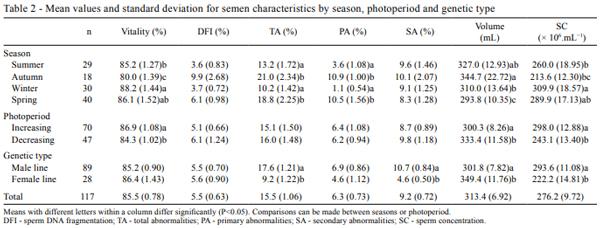
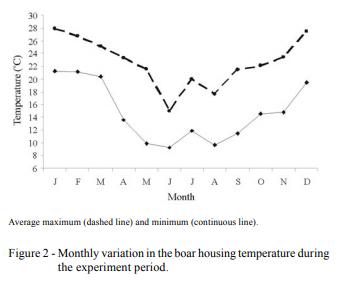
The objective of this study was to evaluate the effects of season, natural photoperiod, and room temperature at the housing facility on boar semen characteristics in Uruguay (34º66'S; 56º29'W). For this purpose, 117 ejaculates, obtained from eight adult males collected through 12 consecutive months, were assessed for sperm viability, DNA integrity, abnormalities (total, primary, and secondary), ejaculate volume, and sperm concentration. Viability, total and primary abnormalities, volume, and sperm concentration were affected by season. Sperm viability, volume, and sperm concentration were affected by natural photoperiod. In general, autumn and the decreasing photoperiod had a negative impact on most of the semen characteristics, except for volume. Housing temperature did not affect semen characteristics. In boars living in temperate climates, semen quality is negatively affected during autumn and is related to photoperiod changes; however, the effects of temperature changes in housingdo not affect these seminal characteristics. In this scenario, seasonal differences in semen quality may have a negative effect on sow fertilization. Consequently, semen quality control especially during autumn is imperative for the best boar selection to be used for insemination purposes. Seasonal differences in semen quality may have a negative effect on sow reproductive performance. This issue will be addressed in a future investigation.
Key Words: boar, natural photoperiod, season, semen quality
Ambrogi, A. 1999. Enfermedades y problemas respiratorios en sistemas al aire libre formas de control. p.67-9. In: 2o Encontro do Conesul de técnicos especialistas em Siscal e 2o Simpósio sobre Siscal. Concórdia, SC, Brasil.
Auvigne, V.; Leneveu, P.; Jehannin, C.; Peltoniemi, O. and Salle, E. 2010. Seasonal infertility in sows: A five year field study to analyze the relative roles of heat stress and photoperiod. Theriogenology 74:60-66.
Avdi, M.; Driancourt, M. A. and Chemineau, P. 1993. Variations saisonnières du comportement d’oestrus et de l’activité ovulatoire chez les brebis Chios et Serres en Grèce. Reproduction Nutrition Développement 33:15-24.
Boe-Hansen, G. B.; Ersbøll, A. K.; Greve, T. and Christensen, P. 2005. Increasing storage time of extended boar semen reduces sperm DNA integrity. Theriogenology 63:2006-2019.
Borg, K. E.; Lunstra, D. D. and Chistenson, R. K. 1993. Semen characteristics, testicular size, and reproductive hormone concentration in mature Duroc, Meishan, Fengjing, and Minzhu boars. Biology Reproduction 49:515-521.
Broekhuijse, M. L. W. J.; Šoštaric, E.; Feitsma, H. and Gadella, B. M. 2012. The value of microscopic semen motility assessment at collection for a commercial artificial insemination centre, a retrospective study on factors explaining variation in pig fertility. Theriogenology 77:1466-1479.
Chemineau, P.; Guillaume, D.; Migaud, M.; Thiéry, J. C.; PellicerRubio, M. T. and Malpaux, B. 2008. Seasonality of reproduction in mammals: intimate regulatory mechanisms and practical implications. Reproduction Domestic Animal 43(Suppl. 2):40-47.
Ciereszko, A.; Ottobre, J. S. and Glogowski, J. 2000. Effects of season and breed on sperm activity and semen quality of boars. Animal Reproduction Science 64:89-96.
Claus, R.; Schopper, D. and Wagner, H-G. 1983. Seasonal effect on steroids in blood plasma and seminal plasma of boars. Journal of Steroid Biochemestry 19:725-729.
De Ambrogi, M.; Spinaci, M.; Galeati, G. and Tamanini, C. 2006. Viability and DNA fragmentation in differently sorted boar spermatozoa. Theriogenology 66:1994-2000.
Delgadillo, J. A.; Leboeuf, B. and Chemineau, P. 1991. Decrease in the seasonality of sexual behavior and sperm production in bucks by exposure to short photoperiodic cycles. Theriogenology 36:755-770.
Elhordoy, D. 1999. Problemas y enfermedades reproductivas más comunes en cerdos de Uruguay. p.78-79. In: 2o Encontro do Conesul de técnicos especialistas em Siscal e 2o Simpósio sobre Siscal. Concórdia, SC, Brasil.
Enciso, M.; López-Fernández, C.; Fernández, J. L.; García, P.; Gosálvez, A. and Gosálvez, J. 2006. A new meted to analyze boar sperm DNA fragmentation under bright-field or fluorescence microscopy. Theriogenology 65:308-316.
Frydrychová, S.; Lustyková, A.; Cerovyský, J.; Lipenský, J. and Rozkot, M. 2007. Seasonal changes of boars semen production. Research Pig Breeding 1:31-33.
Greenberg, L. G. and Mahone, J. P. 1981. The effect of 15-h photoperiod on reproductive function in boars at 2, 3, 4 or 5 months of age. Canadian Journal of Animal Science 61:925-934.
Hanenberg, E. H. A. T.; Knol, E. F. and Merks, J. W. M. 2001. Estimates of genetic parameters for reproductions traits at different parities in Dutch Landrace pigs. Livestock Production Science 69:179-186.
Harayama, H.; Kanda, S. and Kato, S. 1992. Influence of season on characteristics of epididymal and ejaculated semen on Meishan boars. Theriogenology 38:491-500.
Hoffman, B. and Landeck, A. 1999. Testicular endocrine function, seasonality and semen quality of the stallion. Animal Reproduction Science 57:89-98.
Hughes, P. E. and Varley, M. A. 1986. Reproducción del cerdo. Zaragoza, Acriba. Kennedy, B. W. and Wilkins, J. N. 1984. Boar, breed and environmental factors influencing semen characteristics of boars used in artificial insemination. Canadian Journal of Animal Science 64:833-843.
Knecht, D.; Srodon, S.; Szulc, K. and Duzinski, K. 2013. The effect of photoperiod on selected parameters of boar semen. Livestock Science 57:364-371.
Kozdrowski, R. and Dubiel, A. 2004. The effect of season on the properties of wild boar (Sus scrofa L.) semen. Theriogenology 80:281-289.
Kunavongkrit, A.; Suriyasomboom, A.; Lundeheim, N.; Heard, T. W. and Einarsson, S. 2005. Management and sperm production of boars under differing environmental conditions. Theriogenology 63:657-667.
López-Fernández, C.; Crespo, F.; Arroyo, F.; Fernández, J. L.; Arana, P.; Johnston, S. D. and Gosálvez, J. 2007. Dynamics of sperm DNA fragmentation in domestic animals II. The stallion. Theriogenology 68:1240-1250.
López-Fernández, C.; Johnston, S. D.; Gosálbez, A. and Gosálvez, J. 2001. Seasonal changes in sperm DNA fragmentation of MurcianoGranadina goats: The compelling case for dynamic assessment. Small Ruminant Reseaech 100:50-53.
Mauget, R. and Boissin, J. 1987. Seasonal changes in testis weight and testosterone concentration in the European wild boar (Sus scrofa L.). Animal Reproduction Science 13:67-74.
Mazzarri, G.; du Mesnil du Buisson, F. and Ortavant, R. 1970. Acción de la temperatura y de la luz sobre la producción y el poder fecundante de los espermatozoides del verraco. Agronomía Tropical 20:173-184.
Minton, J. E.; Fent, R. W. and Wettemann, R. P. 1985. Influence of the duration of photoperiod on growth, testicular characteristics and endocrine function of boars. Domestic Animal Endocrinology 2:53-59.
Motta, A. 1991. Evaluación de los efectos de la estación y del tipo de servicio sobre la eficiencia reproductiva en una granja porcina del sur del país. Tesis (Grado). UdelaR, Facultad de Agronomía, Montevideo, Uruguay.
Pérez-Pé, R.; Cebrián-Pérez, J. A. and Muiño-Blanco, T. 2001. Semen plasma proteins prevent cold-shock membrane damage to ram spermatozoa. Theriogenology 56:425-434.
Petrocelli, H.; Pérez-Clariget, R.; Franco, J.; Haretche, J.; Burgueño, J. and López, A. 2003. Efecto de la raza, mes de colección y de servicio sobre la calidad seminal de verracos y desempeño al parto de cerdas inseminadas artificialmente. Agrociencia 7:60-67.
Popwell, J. M. and Flowers, W. L. 2004. Variability in relationships between semen quality and estimates of in vivo and in vitro fertility in boars. Animal Reproduction Science 81:97-113.
Rijsselaere, T.; Maes, D.; Hoflack, G.; de Kruif, A. and Van Soom, A. 2007. Effect of body weight, age and breeding history on canine sperm quality parameters measured by the Hamilton-Thorne analyzer. Reproduction Domestic Animal 42:143-148.
Rivera, M. M.; Quintero-Moreno, A.; Barrera, X.; Palomo, M. J.; Rigau, T. and Rodríguez-Gil, J. E. 2005. Natural Mediterranean photoperiod does not affect the main parameters of boar-semen quality analysis. Theriogenology 64:934-946.
Ruiz-Sánchez, A. L.; O’Donoghue, R.; Novak, S.; Dyck, M. K.; Cosgrove, J. R.; Dixon, W. T. and Foxcroft, G. R. 2006. The predictive value of routine semen evaluation and IVF technology for determining relative boar fertility. Theriogenology 66:736-748.
Sancho, S.; Pinart, E.; Briz, M.; Garcia-Gil, N.; Badia, E.; Bassols, J.; Kádár, E.; Pruneda, A.; Bussalleu, E.; Yeste, M.; Coll, M. G. and Bonet, S. 2004. Semen quality of postpubertal boars during increasing and decreasing natural photoperiods. Theriogenology 62:1271-1282.
Sancho, S.; Rodríguez-Gil, J. E.; Pinart, E.; Briz, M.; García-Gil, N.; Badia, E.; Bassols, J.; Pruneda, A.; Bussalleu, R.; Yeste, M.; Casa, I.; Palomo, M. J.; Ramió, L. and Bonet, S. 2006. Effects of exposing boars to different artificial light regimens on semen plasma markers and “in vivo” fertilizing capacity. Theriogenology 65:317-331.
Schopper, D.; Gaus, J.; Claus, R. and Bader, H. 1984. Seasonal changes of steroid concentrations in seminal plasma of a European wild boar. Acta Endocrinology 107:425-427.
Sellés, E.; Gadea, J.; Romar, R.; Matás, C. and Ruiz, S. 2003. Analysis of in vitro fertilizing capacity to evaluate the freezing procedures of boar semen and to predict the subsequent fertility. Reproduction Domestic Animal 38:66-72.
Smital, J. 2009. Effects influencing boar semen. Animal Reproduction Science 110:335-346.
Stone, B. A.; Alex, A.; Werlin, L. B. and Marrs, R. P. 2013. Age thresholds for changes in semen parameters in men. Fertility Sterility 100:952-958.
Tardif, S.; Laforest, J-P.; Cormier, N. and Bailey, J. L. 1999. The importance of porcine sperm parameters on fertility in vivo. Theriogenology 52:447-459.
Thonneau, P.; Bujan, L.; Multigner, L. and Mieusset, R. 1998. Occupational heat exposure and male fertility: a review. Human Reproduction 13:2122-2125.
Trudeau, V. and Sanford, L. M. 1986. Effect of season and social environment on testis size and semen quality of the adult Landrace boar. Journal of Animal Science 63:1211-1219.
Wolf, J. and Smital, J. 2009. Quantification of factors affecting semen traits in artificial insemination boars from animal model analyses. Journal of Animal Science 87:1620-1627.
Wysokinska, A.; Kondracki, S.; Kowalewski, D.; Adamiak, A. and Muczynska, E. 2009. Effect of seasonal factors on the ejaculate properties of crossbred Duroc x Pietrain and Pietrain x Duroc boars as well as purebred Duroc and Pietrain boars. Bulletin of the Veterinary Institute in Pulawy 53:677-685.
Zhang, X. Z.; Liu, J. H.; Sheng, H. Q.; Wu, H. J.; Wu, Y.; Yao, K. S.; Lu, J. C. and Zhang, F. B. 2013. Seasonal variation in semen quality in China. Andrology 1:639-643.






