
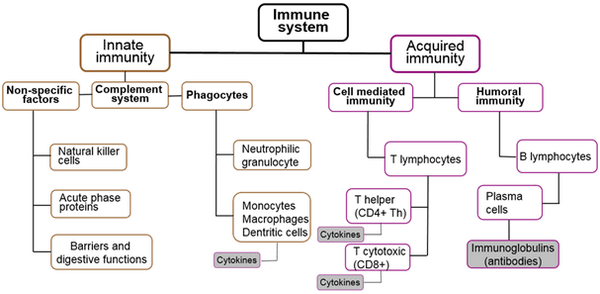
Roles of functional amino acids in the immune system of pigs
- Amino acids are involved in various important metabolic pathways beyond growth, including modulating the functioning of the body’s immune system.
- During a condition of sub-clinical disease, increased dietary supply of functional amino acids (i.e. methionine, tryptophan, threonine, arginine, glutamine, and glycine) ameliorates the negative effect of growth reduction associated with immune challenge.
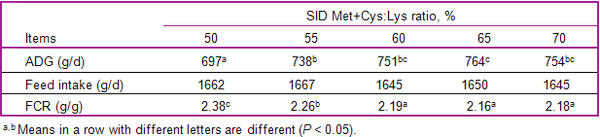
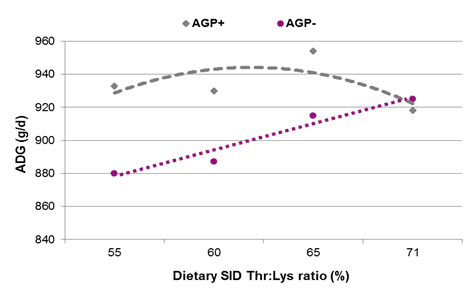
- Based on the available literature, increasing ideal ratios of some functional amino acids – such as the standardized ileal digestibles methionine+cystine:lysine (+ 6%-points), tryptophan:lysine (+ 3%-points), and threonine:lysine (+ 5%-points) – relative to the ratios applied for their healthy counterparts can enhance immune status and optimize growth performance of pigs challenged by sub-clinical disease.
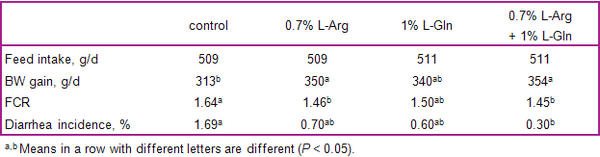
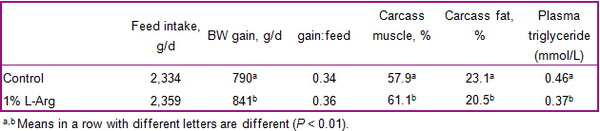
- In pigs with subclinical infections of the gut, increased dietary supply of threonine, arginine, glutamine, and glycine may help enhance immune status and gut integrity.
- Further research is needed to assess the interaction or synergistic effects of these functional amino acids on an animal’s immune function.
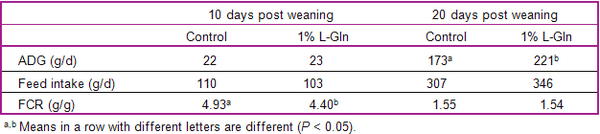


Bertolo, R. F., C. Z. Chen, G. Law, P. B. Pencharz, and R. O. Ball. 1998. Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically. Journal of Nutrition 128:1752-1759.
Bikker, P., J. Fledderus, L. Le Bellego, and M. Rovers. 2007. Growth response of pigs to dietary threonine:lysine ratio is affected by the withdrawal of antimicrobial growth promoters. European Association for Animal Production 124:557-560.
Blecha, F. 2001. Immunology. Pages 688-711 in Biology of the Domestic Pig. W. G. Pond and H. J. Mersmann, ed. Cornell Univ. Press, Ithaca, NY.
Botting, N. P. 1995. Chemistry and neurochemistry of the kynurenine pathway of tryptophan metabolism. Chemical Society Reviews 24:401-412.
Bowland, J. P. 1966. in: L. K. Bustad, R. O. McClellan and M.P. Burns (Ed.): Swine in biomedical research, Frayn, USA.
Capozzalo, M. M., J.C. Kim, J.K. Htoo, C.F.M. de Lange, B.P. Mullan, J.W. Resink, C.F. Hansen, P.A. Stumbles, D.J. Hampson, N. Ferguson, and J.R. Pluske. 2013. A tryptophan:lysine ratio of 0.24 gives optimum performance of weaner pigs under commercial conditions. Manipulating Pig Production XIV. Proceedings of the Fourteenth Biennial Conference of the Australasian Pig Science Association, November 24-27, 2013, Melbourne, Australia. p. 91.
Capozzalo, M. M., J. W. Resink, J. K. Htoo, J. C. Kim, C. F. M. de Lange, and C. F. Hansen. 2014. Optimum sulfur amino acid to lysine ratio for weaner pigs infected with enterotoxigenic E. coli. Journal of Animal Science 92 (Suppl. 2):40. (Abstr.).
Capozzalo, M. M., J. C. Kim, J. K. Htoo, C. F.M. de Lange, B. P. Mullan, C. F. Hansen, J-W. Resink, P. A. Stumbles, D. J. Hampson, and J. R. Pluske. 2015. Effect of increasing the dietary tryptophan to lysine ratio on plasma levels of tryptophan, kynurenine and urea and on production traits in weaner pigs experimentally infected with an enterotoxigenic strain of Escherichia coli. Archives of Animal Nutrition 69(1):17-29.
Chen, H., J. Lin, H. Fung, L. Ho, P. Yang, W. Lee, Y. Lee, and R. Chu. 2003. Serum acute phase proteins and swine health status. Canadian Journal of Veterinary Research 67:283-290.
Colditz, I. G. 2002. Effects of the immune system on metabolism: implications for production and disease resistance in livestock. Livestock Production Science 75:257-268.
Cuaron, J. A., R. P. Chapple, and R. A. Easter. 1984. Effect of lysine and threonine supplementation of sorghum gestation diets on nitrogen balance and plasma constituents in first-litter gilts. Journal of Animal Science 58:631-637.
Cunningham-Rundles, S. (2002): Evaluation of the effects of nutrients on immune function. Pages 57-92 in: Calder P.C., Field C.J., Gill H. S. (eds.) Nutrition and immune function. CABI Publishing. Wallingford, UK.
de Abreu, M. L. T., J. L. Donzele, A. Saraiva, R. F. M. de Oliveira, E. L. Fortes, and G. L. L. Graña. 2010. Glutamine, nucleotides and swine plasma in diets for weaned piglets. Revista Brasileira de Zootecnia 39(3):520-525.
de Jonge, W. J., K. L. Kwikkers, A. A. Te Velde, S. J. H. van Deventer, M. A. Nolte, R. E. Mebius, J. M. Ruijter, M. C. Lamers, and W. H. Lamers. 2002. Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. Journal of Clinical Investigation 110:1539-1548.
de Ridder, K., C. L. Levesque, J. K. Htoo, and C. F. M. de Lange. 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. Journal of Animal Science 90:3485-3491.
Dwyer, D. S. 1979. Regulation of the immune response by polyamines. Medical Hypotheses 5:1169-1181.
Fang, Y. Z., S. Yang, and G. Wu. 2002. Free radicals, antioxidants, and nutrition. Nutrition 8, 872-879.
Galli, F. 2007. Amino acid and protein modification by oxygen and nitrogen species. Amino Acids 32:497-499.
Grimble, R. F. 2002. Sulphur amino acids, glutathione and immune function. Pages 133-150 in: Nutrition and Immune Function; Calder PC, Field CJ, Gill HS. (eds.); CABI Publishing: New York, NY, USA.
Hamard, A., B. Seve, and N. Le Floch. 2007. Intestinal development and growth performance of early-weaned piglets fed a low-threonine diet. Animal 1:1134-1142.
Han, J., Y. L. Liu, W. Fan, J. Chao, Y. Q. Hou, Y. L. Yin, H. L. Zhu, G. Q. Meng, and Z. Q. Che. 2009. Dietary L-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Amino Acids 37:643-651.
Hsu, C. B., S. P. Cheng, J. C. Hsu, and H. T. Yen. 2001. Effect of threonine addition to a low protein diet on IgG levels in body fluid of first litter sows and their piglets. Asian-Australian Journal of Animal Science 14:1157-1163.
Hunter, E. A. and R. F. Grimble. 1994. Cysteine and methionine supplementation modulate the effect of tumour necrosis factor alpha on protein synthesis, glutathione and zinc concentration of liver and lung in rats fed a low protein diet. Journal of Nutrition 124(12):2319-2328.
Jahoor, F., L. Wykes, M. Del Rosario, M. Frazer and P. J. Reeds. 1999. Chronic protein undernutrition and an acute inflammatory stimulus elicit different protein kinetic responses in plasma but not in muscle of piglets. Journal of Nutrition 129:693-699.
Jayaraman, B., J. K. Htoo, and C.M. Nyachoti. 2014. Effects of dietary threonine:lysine ratio and sanitary conditions on performance and plasma urea nitrogen of weaned pigs fed antibiotic-free diets. Journal of Animal Science 92(E-Suppl. 1):218. (Abstr.).
Jayaraman, B., J. K. Htoo,and C.M. Nyachoti. 2015. Effects of increasing standardized ileal digestible tryptophan:lysine ratio on performance and ileal expression of cytokine mRNA in weaned pigs challenged with Escherichia coli K88. Journal of Animal Science 93 (Suppl. S3):201-202. (Abstr.).
Kim, S. W., R. D. Mateo, G. Wu, J. A. Carroll, and I. Shinzato. 2006. Dietary L-arginine supplementation affects immune status of pregnant gilts. Journal of the Federation of American Societies for Experimental Biology. 20:A424.
Kim, S. W., R. D. Mateo, Y. L. Yin, and G. Wu. 2007. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Australasian Journal of Animal Science 20:295-306.
Kim, J. C., B. P. Mullan, B. Frey, H. G. Payne, and J. R. Pluske. 2012. Whole body protein deposition and plasma amino acid profiles in growing and/or finishing pigs fed increasing levels of sulfur amino acids with and without Escherichia coli lipopolysaccharide challenge. Journal of Animal Science 90:362-365.
Kuby, J. 1994. Immunology. 2nd Ed. W. H. Freeman and Company. New York, New York.
Kampman – van de Hoek, E. 2015. Impact of health status on amino acid requirements of growing pigs. Towards feeding strategies for farms differing in health status. PhD thesis. Wageningen University, Wageningen. The Netherlands.
Law, G. K., R. F. Bertolo, A. Adjiri-Awere, P. B. Pencharz, and R. O. Ball. 2007. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. American Journal of Physiology-Gastrointestinal and Liver Physiology 292:G1293-G1301.
Le Floc’h, N. and B. Seve. 2007. Biological roles of tryptophan and its metabolism. Potential implications for pig feeding. Livestock Science 112:23-32.
Le Floc’h, N., D. Melchior, L. Le Bellego, J. J. Matte, and B. Sève. 2007. Does sanitary status have an effect on tryptophan requirement for growth of postweaning piglets? (in French, with English abstract). Journees Rech. Porcine en France 39:125-132.
Le Floc’h, N., D. Melchior, and B. Seve. 2008. Dietary tryptophan helps preserve tryptophan homeostasis in pigs suffering from lung inflammation. Journal of Animal Science 86:3473-3479.
Li, D. F., C. T. Xiao, S. Y. Qiao, J. H. Zhang, E. W. Johnson, and P. A. Thacker. 1999. Effects of dietary threonine on performance, plasma parameters and immune function of growing pigs. Animal Feed Science and Technology 78:179-188.
Li, P., Y-L. Yin, D. Li, S. W. Kim, and G. Wu. 2007. Amino acids and immune function. British Journal of Nutrition 98:237-252.
Lien, K. A., W. C. Sauer, and M. Fenton. 1997. Mucin output in ileal digesta of pigs fed a protein-free diet. Zeitschrift für Ernährungswissenschaft 36:182-190.
Litvak, N., J. K. Htoo, and C. F. M. de Lange. 2013a. Restricting sulfur amino acid intake in growing pigs challenged with lipopolysaccharides decreases plasma protein and albumin synthesis. Canadian Journal of Animal Science 93:505-515.
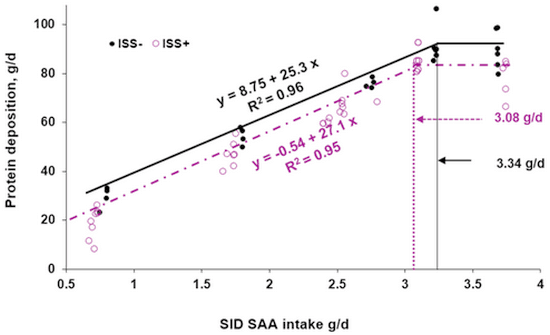
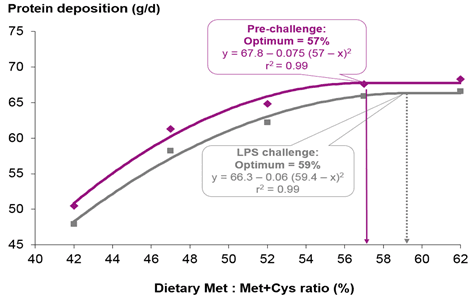
Litvak, N., A. Rakhshandeh, J. K. Htoo, and C. F. M. de Lange. 2013b. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. Journal of Animal Science 91:4188-4196.
Liu, Y., J. Han, J. Huang, X. Wang, F. Wang, and J. Wang. 2009. Dietary L-arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide challenge. Asian-Australasian Journal of Animal Science 22(12):1667-1675.
Liu, T., J. Peng, Y. Xiong, S. Zhou, and X. Cheng. 2002. Effects of dietary glutamine and glutamate supplementation on small intestinal structure, active absorption and DNA, RNA concentrations in skeletal muscle tissue of weaned piglets during d 28 to 42 of age. Asian-Australasian Journal of Animal Science 15(2):238-242.
Lu, S. C. 2009. Regulation of glutathione synthesis. Molecular Aspects of Medicine 30:42-59.
Ma, X., Y. Lin, Z. Jiang, C. Zheng, G. Zhou, D. Yu, T. Cao, J. Wang, and F. Chen. 2010. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 38:95-102.
Moeser, A. J., Klok, C. V., Ryan, K. A., Wooten, J. G., Little, D., Cook, V. L., and A. T. Blikslager. 2007. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. American Journal of Physiology-Gastrointestinal and Liver Physiology 292:G173-G181.
Molino, J. P., J. L. Donzele, R. F. M. de Oliveira, A. Saraiva, D. Haese, E. L. Fortes, and M. F. de Sauza. 2012. L-glutamine and L-glutamate in diets with different lactose levels for piglets weaned at 21 days of age. Revista Brasileira de Zootecnia 41(1):98-105.
Myrie, S. B., R. F. Bertolo, W. C. Sauer, and R. O. Ball. 2008. Effect of common antinutritive factors and fibrous feedstuffs in pig diets on amino acid digestibilities with special emphasis on threonine. Journal of Animal Science 86:609-619.
Mathai, J. K., J. K. Htoo, J. Thomson, K. J. Touchette, and H. H. Stein. 2015. Effects of dietary fiber on the optimum threonine:lysine ratio for 25- to 50-kg gilts. Journal of Animal Science 93(Suppl. s3):298. (Abstr.).
Mayer, L. 2000. Mucosal immunity and gastrointestinal antigen processing. Journal of Pediatric Gastroenterology and Nutrition 30(Suppl.):S4-S12.
Melchior, D., B. Sève, and N. Le Floc’h. 2004. Chronic lung inflammation affects plasma amino acid concentrations in pigs. Journal of Animal Science 82:1091-1099.
NRC, 2012. Nutrient Requirements of Swine. 11th ed., Washington D.C. USA.
Obled, C. 2003. Amino acid requirements in inflammatory states. Canadian Journal of Animal Science 83(3):365-373.
Oswald, I. P. 2006. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Veterinary Research 37:359-368.
Parkin, J. and B. Cohen. 2001. An overview of the immune system. Lancet 357:1777-1789.
Pastorelli, H., J. van Milgen, P. Lovatto, and L. Montagne. 2012. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952-961.
Pluske, J. R., Hampson, D. J., and I. H. Williams. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livestock Production Science 51:215-236.
Powell, S., T. B. Bidner, R. L. Payne, and L. L. Southern. 2011. Growth performance of 20- to 50-kilogram pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. Journal of Animal Science 89:3643-3650.
Rakhshandeh, A., J. K. Htoo, and C. F. M. de Lange. 2010. Immune system stimulation of growing pigs does not alter apparent ileal amino acid digestibility but reduces the ratio between whole body nitrogen and sulfur retention. Livestock Science 134:21-23.
Rakhshandeh, A., J. K. Htoo, N. Karrow, S. P. Miller, and C. F. M. de Lange. 2014. Impact of immune system stimulation on ileal nutrient digestibility and utilization of methionine plus cysteine intake for whole body protein deposition in growing pigs. British Journal of Nutrition 111:101-110.
Reeds, P. J., D. G. Burrin, B. Stoll, F. Jahoor, L. Wykes, J. Henry, and M. E. Frazer. 1997. Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. American Journal of Physiology 273:E408-E415.
Reeds, P. J. and F. Jahoor. 2001. The amino acid requirements of disease. Clinical Nutrition 20 (S1):15-22.
Roth, E. 2007. Immune and cell modulation by amino acids. Clinical Nutrition 26:535-544.
Shao L., Serrano D. and L. Mayer. 2001: The role of epithelial cells in immune regulation in the gut. Seminars in Immunology 13:163-176.
Shan, Y., A. Shan, J. Li, and C. Zhou. 2012. Dietary supplementation of arginine and glutamine enhances the growth and intestinal mucosa development of weaned piglets. Livestock Science 150:369–373.
Stadnyk A.W. 2002. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines, Canadian Journal of Gastroenterology 16:241-246.
Stoll, B. and D. G. Burrin. 2006. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. Journal of Animal Science 84(Suppl. 13):E60-72.
Stoll, B., J. Henry, P. J. Reeds, H. Yu, F. Jahoor, and D. G. Burrin. 1998. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. Journal of Nutrition 128:606-614.
Stipanuk, M. H. and I. Ueki. 2011. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. Journal of Inherited Metabolic Diseases 34(1):17-32.
Strous, G. J. and J. Dekker. 1992. Mucin-type glycoproteins. Critical review in Biochemistry and Biology 27:57-92.
Tan B., X.G. Li, X. Kong, R. Huang, Z. Ruan, K. Yao, Z. Deng, M. Xie, I. Shinzato, Y. Yin, and G. Wu. 2009a. Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 37:323-331.
Tan, B., Yin Y., Z. Liu, X. Li., H. Xu., X. Kong, R. Huang, W. Tang, I. Shinzato, S. B. Smith, and G. Wu. 2009b. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37:169-175.
Tan, B., M. Xie and Y. Yin. 2013. Amino acids and immune functions. Pages 175-185 in: Nutritional and Physiological Functions of Amino Acids in Pigs. Eds. F. Blachier, G. Wu and Y. Yin. Heidelberg, Germany: Springer.
Tate, S. S. and A. Meister. 1971. Regulation of rat liver glutamine synthase: Activation by α-ketoglutarate and inhibition by glycine, alanine, and carbamyl phosphate. Proceedings of the National Academy of Sciences of the United States of America 68:781-785.
Visek, W. J. 1986. Arginine needs, physiological states and usual diets. A reevaluation. Journal of Nutrition 116:36-46.
Wang, X., S. Y. Qiao, M. Liu, and Y. X. Ma. 2006. Effects of graded levels of true ileal digestible threonine on performance, serum parameters and immune function of 10-25 kg pigs. Animal Feed Science and Technology 129:264-278.
Wang, X., S. Y. Qiao, Y. L. Yin, L. Yue, Z. Wang, and G. Wu. 2007. A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs. Journal of Nutrition 137:1442-1446.
Wang, J. J., L. X. Chen, P. Li, X. L. Li, H. J. Zhou, F. L. Wang, D. F. Li , Y. L. Yin, and G. Wu. 2008. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. Journal of Nutrition 138:1025-1032.
Wang, W., Z. Wu., G. Lin., S. Hu, B. Wang, Z. Dai, and G. Wu. 2014. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. Journal of Nutrition 144(10):1540-1548.
Widner, B., M. Ledochowski, and D. Fuchs. 2000. Interferon-gamma induced tryptophan degradation: neuropsychiatric and immunological consequences. Current Drugs Metabolism 1:193-204.
Wu, G., S. A. Meier, and D. A. Knabe. 1996. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. Journal of Nutrition 126:2578-2584.
Wu, G. 1998. Intestinal mucosal amino acid catabolism. Journal of Nutrition 128:1249-1252.
Wu, G. Y. and S. M. Jr. Morris. 1998. Arginine metabolism: nitric oxide and beyond. Biochemical Journal 336:1-17.
Wu, G., Y. Fang, S. Yang, J. R. Lupton, and N. D. Turner. 2004. Glutathione metabolism and its implications for health. Journal of Nutrition 134:489-492.
Wu, G., W. B. Fuller, T. A. Davis, L. A. Jaeger, G. A. Johnson, S. W. Kim, D. A. Knabe, C. J. Meininger, T. E. Spencer, and Y-L Yin. 2007. Important roles for the arginine family of amino acids in swine nutrition and production. Livestock Science 112:8-22.
Wu, G. 2009. Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1-17.
Wu, G. 2010. Functional amino acids in growth, reproduction, and health. Advances in Nutrition 1:31-37.
Wu, X., Z. Ruan, Y. Gao, Y. Yin, X. Zhou, L. Wang, M. Geng, Y. Hou, and G. Wu. 2010. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39:831-839.
Yao K, Y. L. Yin, W. Y. Chu, Z. Liu, D. Deng, T. Li, R. Huang, J. Zhang, B. Tan, W. Wang, and G. Wu. 2008. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. Journal of Nutrition 138:867-872.
Yoneda, J., A. Andou, and K. Takehana. 2009. Regulatory roles of amino acids in immune response. Current Rheumatology Reviews 5:252-258.
Yoo, S. S., C. J. Field, and M. I. McBurney. 1997. Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. Journal of Nutrition 127:2253-2259.
Zhang, G. J., Q. L. Song, C. Y. Xie, L. C. Chu, P. A. Thacker, J. K. Htoo, and S. Y. Qiao. 2012. Estimation of the ideal ratio of standardized ileal digestible tryptophan to lysine for growing pigs fed low crude protein diets supplemented with crystalline amino acids. Livestock Science 149:260-266.
Zhang, G. J., P.A. Thacker, J.K. Htoo, and S.Y. Qiao. 2015. Optimum proportion of standardized ileal digestible sulfur amino acid to lysine to maximize the performance of 25–50 kg growing pigs fed reduced crude protein diets fortified with amino acids. Czech Journal of Animal Science 60(7):302-310.
Zhong, Z., M. D. Wheeler, X. L. Li, M. Froh, P. Schemmer, M. Yin, H. Bunzendaul, B. Bradford, and J. J. Lemasters. 2003. Glycine: a novel anti-inflammatory, immunomodulatory, and cytoprotective agent. Current Opinion in Clinical Nutrition and Metabolic Care 6:229-240.
Zhu, C. L., M. Rademacher, and C. F. M. de Lange. 2005. Increasing dietary pectin level reduces utilization of digestible threonine intake, but not lysine intake, for body protein deposition in growing pigs. Journal of Animal Science 83:1044-1053.
Zou, X. T., G. H. Zheng, X. K. J. Fang, and J. F. Fang. 2006. Effects of glutamine on growth performance of weanling piglets. Czech Journal of Animal Science 51(10):444-448.



.jpg&w=3840&q=75)