Practical Dietary Approaches to Increase Longissimus Intramuscular Fat Content and Their Influence on Glycolytic Potential and Pork Quality Characteristics
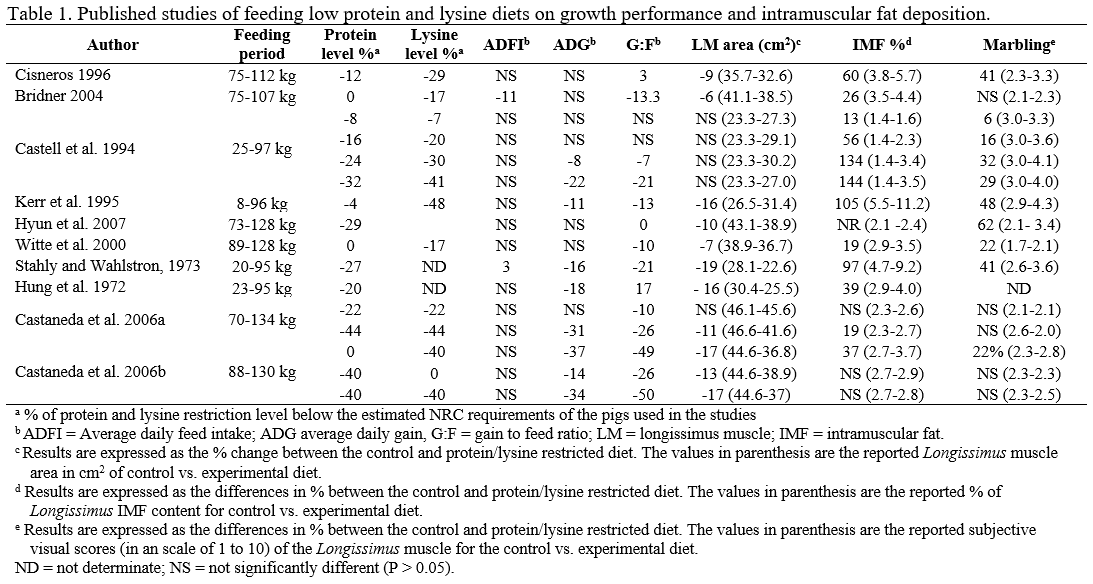
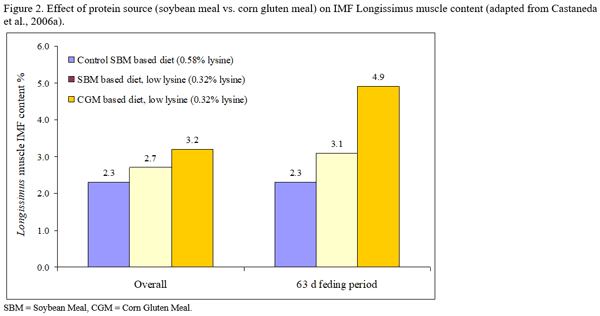
- Low lysine diets. In general, the greatest the lysine restriction the greater the increase in IMF content. Different dietary approaches have been used to achieve the protein and lysine restriction levels, in those cases were the lysine restriction level (0.40% of lysine level or below) results in soybean meal inclusion levels of 7% or below, total crude protein and other essential amino acids (lysine, valine, histidine, and isoleucine) (Russell et al., 1993; Figueroa et al., 2003) may be limiting. Further research is necessary to understand the combined and independent effects of those nutrients in order to improve the formulation strategies to achieve the response in IMF content.
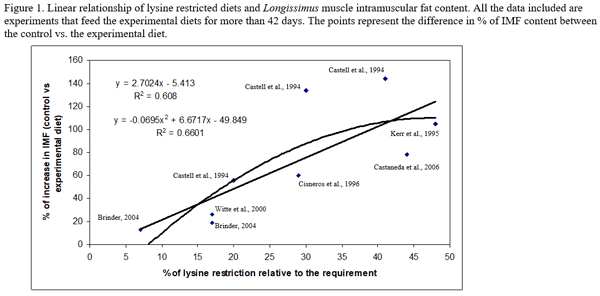
- Time on feed. From the research reported in this review, we can conclude that the degree of lysine restriction interact with time on feed (Castaneda et al., 2005a). In theory as the degree of lysine restriction and the time on feed increase, the magnitude of the response in IMF will also increase. From this literature review, it is difficult to clearly establish this relationship, more research is necessary to fully understand the relationship of low protein diets and the period of time in which those diets are feed and their impact on IMF.
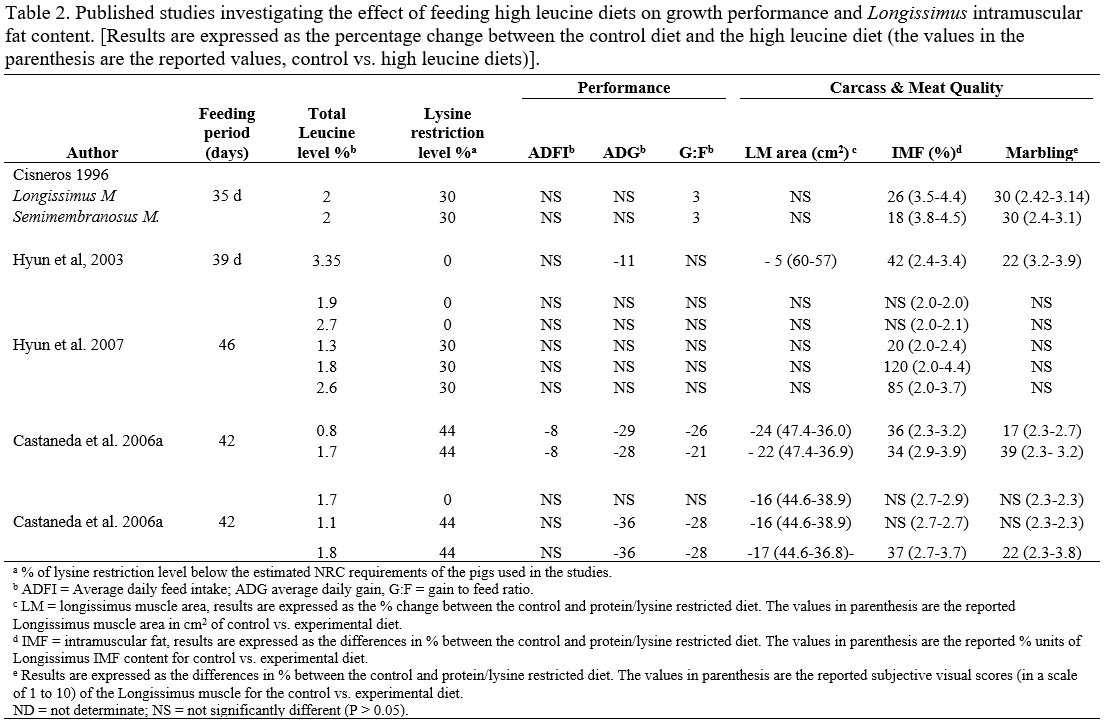
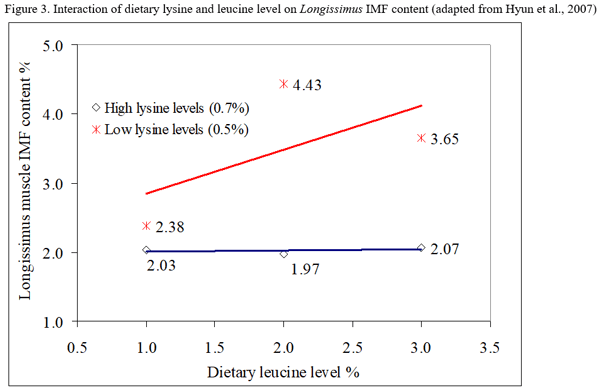
- Lysine-leucine interaction. There has been limited research to test for interactions between high dietary leucine levels and low dietary lysine levels. Both nutrients operate by increasing the availability of energy for fat deposition within the muscle, therefore the degree of restriction of one nutrient may an important factor for the expression of the other on IMF content. More research is needed to understand the combined effects of these two approaches.
- High dietary leucine levels and glycogen replenishment. An excess of dietary leucine may induce a delay in glycogen replenishment during or after muscle depletion because an excess of this amino acid can contribute to the energy supply at the muscle level. However, this hypothesis has never been evaluated.
- We hypothesize that the deposition of intramuscular fat in different genotypes of finishing pig would be a function of the protein-lysine intake and the growth potential of those genotypes. The highest level of intramuscular fat deposition will be reached when the fattest genotype feed the low lysine diets.
- We hypothesize that different approaches to reduce dietary lysine (which loads different levels of protein, lysine and other essential amino acids) will have different impact on IMF content. The highest level of IMF content will be reached with pigs fed the lowest soybean meal level due to a low protein and lysine intake and imbalances in other amino acids.
- We hypothesize that in the absence of any negative effect of dietary protein-lysine restriction on feed intake (i.e. energy intake), the response in IMF would increase with increasing degree of protein-lysine restriction.
- We hypothesize that as the degree of lysine restriction and the time on feed increase, the magnitude of the response in IMF will also increase.
- We hypothesize that the deposition of intramuscular fat in the finishing pig would be a function of both lysine and leucine intake. The highest level of intramuscular fat deposition will be reached when the optimum inclusion levels for lysine and leucine are achieved.
- We also hypothesize that an excess of leucine may induce a delay in glycogen replenishment during or after muscle depletion.
Andersen, H. J., N. Oksbjerg, J. F. Young, and M. Therkildsen. 2005. Feeding and meat quality-a future approach. Meat Sc. 70:543-554
AOAC. 1998. Official Methods and recommended practices of the AOAC. 5th ed. Am. Oil. Chem. Soc. Champaign, Il.
Baker, D. H. 1993. Ideal Protein for Pigs. Manipulating Pig Production IV. Proc.Biennial Conf. Australasian Pig Science Association. Camberra, Australia. 191
Baker, D. H. and T. K. Chung. 1992. Ideal protein for swine and poultry. BioKyowa Tech. Rev. 4
Bellego, L., J. van Milgen, S. Dubois and J. Noblet. 2001. Energy utilization of low-protein diets in growing igs. J. Anim. Sci. 79:1259-1271
Bertol, T. M. 2003. Management and nutritional approaches to reduce glycolytic potential and stress responses in pigs. PhD Diss. University of Illinois. Urbana. Illinois
Bertol, T. M., M. Ellis, M. J. Ritter, F.K. Mckeith, and D. N. Hamilton. 2005. Variation in glycolytic potential and fresh pork quality traits along the longissimus dorsi of slaughter weight pigs. J Muscle Foods. 17:237-247
Brewer, M. S., L. G. Zhu, and F. K. Mckeith. 2001. Marbling effects on quality characteristics. Meat Sci. 59: 153-163
Calvert, C.C., K. C. Klasing, and R.E. Austric. 1992. Involvement of food intake and amino acid catabolism in the branched chain amino acid antagonism in chicks. Journal of Nutrition. 112:627-635
Castaneda, E. O. S. 2005a. Nutritional approaches to increase intramuscular fat in pigs. Chapter IV. The impact of time of feeding of lysine-deficient diets and dietary protein level on the intramuscular fat content of pork. PhD Diss. University of Illinois. Urbana. Illinois
Castaneda, E. O. S. 2005b. Nutritional approaches to increase intramuscular fat in pigs. Chapter V. The impact of Level of dietary crude protein and lysine, and protein source on intramuscular fat and pork quality. PhD Diss. University of Illinois. Urbana. Illinois
Castell, A. G., R. L. Cliplef, L.M. Poste-Flynn, and G. Butler. 1994. Performance, carcass and pork characteristics of castrates and gilts self-fed does differing in protein content and lysine:enery ratio. Can J. Anim. Sci. 74:519-528
Chang, K., N. da Costa, R. Blacley, O. Southwood, G Evans, G Plastow, J. D. Wood, and R. I. Richardson. 2003. Relationship of myosyn heavy chain fiber types to meat quality traits in traditional and modern pigs. Meat. Sci. 64:93-103
Cisneros, F., M. Ellis, D. H. Baker, R. A. Easter, and F. K. Mckeith. 1996. The influence of short-term feeding of amino acid-deficient diets and high dietary leucine levels on the intramuscular fat content of pig muscle. Br. Soc. Anim. Sci.. 63:517-522
DeVol, D. L., F. K. McKeith, P. J. Bechtel, J. Novakovsky, R. D. Shanks, and T. D. Carr. 1988. Variation in composition and palatability traits and relationship between muscle characteristics and palatability in a random sample of pork carcasses. J. Anim. Sci. 66: 385-395.
D'Mello J. P. F., and Lewis D. 1970. Amino acid interaction in chicks nutrition. 1. The interrelationship between leucine, isoleucine and valine. British Poultry Science. 11:299-311
D'Mello, 2003, Amino acids in animal nutrition. Second edition. CABI Publishing. CAB International.
D'Mello, 2003, Amino acids in animal nutrition. Second edition. CABI Publishing. CAB International.
D'Souza, D. N., D. W. Pethick, F. R. Dunshea, D. Suster, J. R. Pluske, and B. P. Mullan. 2004. The pattern of fat deposition differs in different pork primal cuts of female pigs during the finisher growth phase. Livest. Prod. Sci. 91: 1-8.
Edmonds M. S., and D. H. Baker. 1987. Amino acids excesses for young pigs: effects of excess methionine, tryptophan, threonine or leucine. J. Anim. Sci. 64:1664-1671
Edmonds M. S., and D. H. Baker. 1987. Comparative effects of individual amino acids excess when added to a corn-soybean meal diet: effects on growth and dietary choice in the chick. J. Anim. Sci. 65:699-705
Ellis, M., A. J. Webb, P. J. Avery, and I. Brown. 1996. The influence of terminal sire genotype, sex, slaughter weight, feeding regimen and slaughter-house on growth performance and carcass and meat quality in pigs and on the organoleptic properties of fresh pork. Animal Science 62:521-530.
Ellis, M., E. Castaneda, M. Ritter, and F. K. McKeith. 2004. Modern trends and their influence on meat quality in swine. Pig and poultry meat quality-genetic and non-genetic factors. Proc. Br. Soc. Anim. Sci. Krakov, Poland.
Fernandez, X., G. Monin, A. Tamant, J. Mourot, and B. Lebret. 1999b. Influence of intramuscular fat content on the quality of pig meat. 2. Consumers acceptability of muscle Longissimus lumborum. Meat Sci. 53: 67-72.
Figueroa, J. L., A. J. Lewis, P. S. Miller, R. L. Fischer, and R. M. Diedrichsen. 2003. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine and valine. J. Anim. Sci. 81:1529-1537
Garret R. H., and Grisham C. M. 2002. Principles of Biochemistry with a human focus. Brooks/Cole. First edition.
Hamilton, D. N., K. D. Miller, M. Ellis, F. K. McKeith, and E. R. Wilson. 2003. Relationships between longissimus glycolytic potential and swine growth performance, carcass traits, and pork quality. J. Anim Sci 81:2206-2212.
Hamilton, D. N., M. Ellis, K. D. Miller, F. K. McKeith, and D. F. Parrett. 2000. The effect of the Halothane and Rendement Napole genes on carcass and meat quality characteristics of pigs. J. Anim Sci 78:2862-2867.
Harper, A. E., R. H. Miller, and K. P. Block. 1984. Branched-chain amino acid metabolism. Annual review of nutrition. 4:409-454
Hartschuh, J.K., J. Novakofski, F.K. McKeith. 2002. Practical aspects of the glycolytic potential assay. Rec. Meat Conf. Proc., 55:39-42.
Honikel, K. O. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49:477-457
Hyun, Y., J. D. Kim, M. Ellis, B. A. Peterson, D. H. Baker, and F. K. Mckeith. 2007. Effect of dietary leucine and lysine levels on intramuscular fat content in finishing pigs. Can. J. Anim. Sci. 87:303-306
Hyun, Y., M. Ellis, F. K. Mckeith, and D. H. Baker. 2003. Effect of dietary leucine level on growth performance and carcas and meat quality in finishing pigs. Can. J. Anim. Sci. 83:315-318
Keppler, D., K. Decker. 1974. Glycogen: Determination with Amyloglucosidase. In: Methods of enzymatic analysis, Vol II. 3:1127.
Kerr, B. J., F. K. McKeith, and R. A. Easter. 1995. Effect on performance and carcass characteristics of nursery to finisher pigs fed reduced crude protein, amino acid-supplemented diets. J. Anim Sci 73:433-440.
Klindt, J., R. M. Thallman, and T. Wise. 2006. Effects of sire line, sire, and sex on plasma urea nitrogen, body weight, and back fat thickness in offspring of Duroc and Landrace boars. J. Anim. Sci. 84:1323-1330
Knowles, T. A., L. L. Southern, T. D. Bridner, B. J. Kerr, and K. G. Friesner. 1998. Effect of dietary fiber of fat in low-crude protein crystalline amino acid-supplementation diets for finishing pigs. J. Anim. Sci. 76:2818-2832
Latorre, M. A., R. Lazaro, M. I. Gracia, M. Nieto, and G. G. Mateos. 2003. Effect of sex and terminal sire genotype on performance, carcass characteristics, and meat quality of pigs slaughtered at 117 kg body weight. Meat Sci 65:1369-1377.
Monin, G., P. Sellier. 1985. Pork of low technological quality with a normal rate of muscle pH fall in the post-mortem period: the case of the Hampshire breed. Meat Sci., 13:49-63.
Murray, A. C. 2002. How much should pig muscle contains? Advances in Pork Production.
Noblet, J., Y. Henry, and S. Dubois. 1987. Effect of protein and lysine levels in the diet on body gain composition and energy utilization in growing pigs. J. Anim. Sci. 65:717-726
Novakovsky, J. S., S. Park, P. J. Bechtel, and F. K. McKeith. 1989. Composition of cooked pork chops: effect of removing subcutaneous fat before cooking. J. Food. Sci. 54: 15-17.
NPPC. 1991. Procedures to Evaluate Market Hog Performance (2nd Ed.). National Pork Producers Council, Des Moines, IA.
NRC. 1998. The Nutrient Requirements of Swine.10th ed, in National Research Council. National Academy Press, Washington. 1-189.
Peter, C. M., Y. Han, S. D. Boling-Frankenbach, C. M. Parsons, and D. H. Baker. 2000. Limiting order of amino acids and the effect of phytase on protein quality in corn gluten meal fed to young chicks. J. Anim. Sci. 78:2150-2156
Rincker, R. J., J. Killefer, M. Ellis, M. S. Brewer, and F. K. McKeith. 2007. Intramuscular fat content has little influence on the eating quality of fresh pork loin chops. J. Anim Sci. 2008. 86:730-737.
Ritter, M. J., M. Ellis, D. B. Anderson, S. E. Curtis, K. K. Keffaber, J. Killefer, F. K. McKeith, C. M. Murphy, and B. A. Peterson. 2009. Effects of multiple concurrent stressors on rectal temperature, blood acid-base status, and longissimus muscle glycolytic potential in market-weight pigs. J. Anim Sci 87:351-362.
Rosenvold, K., B. Essen-Gustavsson, and H. J. Andersen. 2003. Dietary manipulation of pro- and macroglycogen in porcine skeletal muscle. J. Anim Sci 81:130-134.
Russel, L. E., G. L. Cromwell, and T. S. Stahly. 1983. Tryptophan, threonine, isoleucine, and methionine supplementation of a 12% protein, lysine-supplemented, corn*soybean meal diet for growing pigs. J. Anim. Sci. 56:[5]1115-1123
Russel, L. E., N. J. Kerr, and Easter. 1987. Limiting amino acids in an 11% crude protein corn-soybean meal diet for growing pigs. J. Anim. Sci. 65:1266-1272
Suzuki, K., M Irie., H. Kadowaki, T. Shibata, M. Kumagai, and A. Nishida. 2005. Genetic parameter estimates of meat quality traits in Durock pigs selected for average daily gain, Longissimus muscle area, back fat thickness, and intramuscular fat content. J. Anim. Sci. 83:2058-2065
van Milgen, J., J. Noblet, S. Dubois. 2001. Energetic efficiency of starch, protein and lipid utilization in growing pigs. J. Nutr., 131:1309-1318.
Witte, D. P., M. Ellis, F. K. McKeith, and E. R. Wilson. 2000. Effect of dietary lysine level and environmental temperature during the finishing phase on the intramuscular fat content of pork. J. Anim Sci 78:1272-1276.
Alvaro Rojo Gomez, I congratulate the authors for an excellent and timely review. As I implemented a research program with pigs of different sexes, aiming to evaluate nutritional programs with variation in the levels of digestible lysine (LD) for pigs from 65 to 160 days, therefore slaughtered with body weight above 100 Kg. I obtained data that confirm that rations with LD levels, below that recommended for different weight ranges, resulting in a greater amount of intramuscular fat, without impairing the animals' performance and carcass. Two of these studies were even published here at ENGORMIX, with the respective titles of Lysine requirement for growing-finishing immunocastrated male pigs and Nutritional plans of digestible lysine for growing-finishing gilts.
In addition to these works, a summary was also published with considerations on the results of this research program under the title; Reducing the cost of pig production is possible, where there is information about the fat content of the animals' muscle. In the opportunity, I associated the increase in intramuscular fat due to the possible increase in the enzyme fatty acid synthetase (FAs) and reduction in the activity of the muscle sensitive hormone lipase (HSL) enzyme caused by the lower level of LD.