Nucleotides from yeast extrac in food animal diets
Nucleotides from yeast extract: potential to replace animal protein sources in food animal diets
For years animal proteins have been used in livestock diets without many questions; however fishmeal, meat and bone meal, blood meal, blood plasma and even milk by-products in some cases are now coming under public scrutiny. This is in part due to a lack of public confidence stemming from appearance of BSE and other disease scares along with salmonella and E. coli contamination of animal food products.
It must be remembered that the majority of the public are very far removed from agriculture production of any kind. This creates a lack of awareness of standard practices in food production, and increases susceptibility to misinformation. If the consumer becomes afraid of certain categories of food, pressure is applied to the food producer to change production practices to methods with which the public is more comfortable.
In many parts of the world some or all of animal protein sources have been banned for use in feeding farm animals. Concerns range from possible routes for transmission of salmonella and E. coli, to transmission of species-specific diseases via the feeding of by-products back to the species of origin, to dioxin contamination, to over-fishing of stocks for fish meal, and even to existing shortages of animal products like milk powders for human consumption.
Producers are asking questions as to how to eliminate possible hygiene breaches in their production systems that might result from utilizing contaminated feed sources, of which animal proteins seem to pose more risk than grain sources, at least with regard to infectious diseases. The net result is an ongoing search for proteins that can replace the nutritional or functional gaps left by the withdrawal of the animal proteins.
Given widespread interest in use of non-animal proteins for feeds, Alltech has introduced a new ingredient based on yeast extract called NuPro. NuPro is a vegetable protein source derived from yeast cell contents. NuPro is rich in inositol, a potential natural growth promoter, glutamate, which has a beneficial impact on palatability, and nucleotides, which offer important nutritional implications for both humans and livestock. The potential of nucleotides via NuPro for dietary supplementation will be discussed herein.
Responses to nucleotides
Recent advances in feed additive research have opened the door to the possibility of utilizing yeast extract, the cell contents of yeast, in animal feeding to fill the void left by the loss of animal proteins. Yeast extract, rich in nucleotides, is traditionally an ingredient used only in human foods. Availability at a reasonable cost and an understanding of potential roles have been the primary limiting factors to its use in animal feeding, but the changing landscape of livestock nutrition is prompting researchers to explore new applications for ingredients like yeast extract. At the same time, other production systems have increased availability of yeast extract.
Findings from the human medical and nutrition research fields indicate a number of possible areas of application (Table 1). Carver and Walker (1995) extensively reviewed the role of nucleotides in human nutrition. The potential beneficial effects upon the immune system, small intestine growth and development, lipid metabolism and hepatic function were examined. The possibility of including supplemental nucleotides in livestock diets for similar reasons is therefore not a new concept, but using yeast extract as the delivery vehicle is a new development.
Table 1. Summary of beneficial properties of nucleotides.
• Improved energy metabolism.
• Improved nitrogen metabolism.
• Improved intestinal morphology.
• Improved growth rate.
• Improved immune response.
• Optimization of the function of fast growing tissues.
• Enhanced maturation rate of villi.
• Flavoring agent, improved palatability.
• Reduced intestinal disorders.
Applications for yeast extract nucleotides
PERFORMANCE RESPONSES IN PIGS
An introductory series of trials exploring the potential of products containing yeast extract and peptides to replace plasma has been previously summarized (Tibbetts, 2000). University and field trials showed equal or better performance when uniformly balanced diets were fed. Among the findings was the fact that although plasma proteins derived from cattle or pigs are a very effective intake enhancer in most trials, performance in terms of growth and feed efficiency did not always follow the improved intake, especially if piglets were disease-challenged.
Mahan (1999) demonstrated that peptide protein products in combination with yeast extract could completely replace plasma protein if diets were correctly formulated. In diets not containing blood plasma, these vegetable proteins have been shown to improve feed intake against controls and other animal protein sources (Maribo, 2001).
The presence of peptides offers improvements in efficiency of utilization of the dietary components, along with the potential for improving intestinal morphology and other physiological changes (Power and Murphy, 1999; Tibbetts, 2000).
Nucleotides improved intestinal growth and maturation (Tsujinaka, 1999) in studies with rats, which could have important implications for post-weaning production stages of various species of farm animals. Often a post-weaning lag period in growth is seen in livestock species in which intestinal villi atrophy and feed utilization is impaired. A shorter villus atrophy period would allow animals to reach peak growth and efficiency more quickly.
Researchers at the University of Missouri (Touchette et al., 1999) reported that spray-dried blood plasma improved intestinal health in postweaning piglets by increasing villus height and villus height:crypt depth ratio regardless of feed intake.
This was thought to be similar to improved pig performance seen with plasma inclusion in earlyweaned piglet diets. Boren et al. (2001), also at the University of Missouri, followed a series of nursery feeding trials with extensive intestinal morphology measurements to investigate effects of vegetable proteins containing yeast extract and peptides in piglets.
A similar improvement in villus height:crypt depth ratio was reported. Cross-sectional area of the lamina propria and villus width were significantly less than the plasma treatment. Importantly, improving gut health in nursery pigs with nucleotiderich yeast extract protein was comparable to improvements noted with plasma and provided long term improvements in growth rate to growing and finishing pigs (Carlson et al., 2001).
Carlson and co-workers (Boren et al., 2001; Carlson et al., 2001) tested spray-dried porcine plasma and vegetable protein sources containing peptides and yeast extract (PYE) in diets for growing pigs on performance and intestinal morphology. Diets were simple corn/soy/wheybased nursery formulations with plasma or PYE being incorporated at 5% for the first two weeks post-weaning in the treatment diets, and 2.5% for the third and fourth weeks post-weaning (Table 2). The nursery performance trials were conducted with and without antibiotics to determine which protein source was more effective (Tables 3 and 4).
Although some differences might have been expected when antibiotics were not used, because apparently healthy pigs were tested in non-continuous flow empty buildings, the inclusion or absence of antibiotics had little effect in these trials on performance. Pigs were started in both trials at approximately 3 weeks of age.
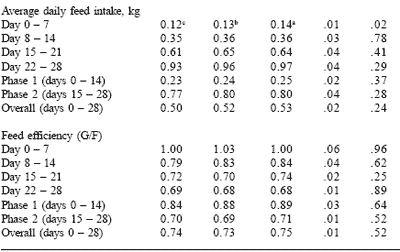
In both trials, growth rate was significantly improved by the inclusion of either plasma or PYE protein source. Plasma and PYE treatments gave similar performance for average daily gain, feed intake and gain to feed ratios (Tables 3 and 4). Feed intake and consequently efficiency were slightly improved in the non-antibiotic trial.
Pigs from the three treatment groups were sacrificed and intestinal morphology examined on days 7, 14 and 28. No significant differences were observed in pigs examined at days 7 or 14. The inclusion of plasma had been previously shown to have an effect on intestinal morphology in the first week post-weaning (Touchette et al., 1999). Lack of response in this study may have been due to the clean health status of the pigs at this time. At day 28, the inclusion of either plasma or PYE proteins improved crypt depth and total wall thickness compared to the controls (Table 5). Including PYE proteins in the nursery diets improved villus width and lamina propria area compared to control and plasma treatments.
Table 2. Percentage composition of experimental basal diets (DM basis).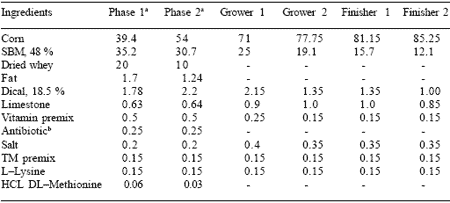
aPlasma or peptide protein (PYE) was added in place of soybean meal in the Phase 1 and Phase 2 nursery diets. Phase 1 diet was formulated
to contain 1.5% lysine, 0.42% methionine, and 0.27% tryptophan and Phase 2 diet was formulated to contain 1.3% lysine, 0.36% methionine, and 0.24% tryptophan.
bExp. 1 only, replaced with corn in Exp. 2.
Carlson et al., 2001
Table 3. Effect of feeding plasma or peptide (PYE) proteins with the addition of Carbadox on nursery pig growth performance.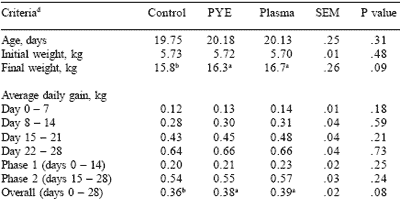
a,b,cMeans in the same row lacking a common superscript differ, (P<0.05).
dLeast square means are reported from 8 pens per treatment.
Carlson et al., 2001.
Table 4. Effect of feeding plasma or peptide (PYE) proteins without a feed grade antibiotic on nursery pig growth performance.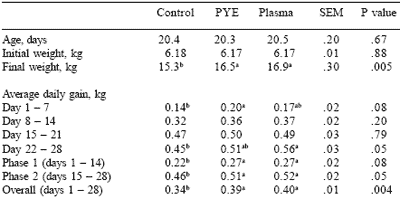
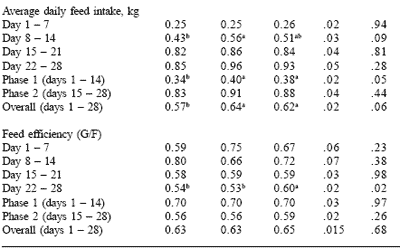
a,bMeans in the same row lacking a common superscript differ (P<0.05).
cLeast square means are reported from 7 pens per treatment.
Carlson et al., 2001.
A final trial in this series at the University of Missouri was conducted to try to evaluate the effect of the morphological changes noted during the nursery phase on finishing performance. Growth rate, feed intake, efficiency of feed conversion and carcass measurements were recorded. Improvement in growth rate in the nursery is commonly thought to result in even further improvements in finishing growth performance.
A rule of thumb is that for every unit of improvement in pig weight at the end of the nursery phase, an additional 2 to 3 units would be noted at the end of the finishing phase. Therefore, it would be expected that finished pigs from both the plasma and PYE treatment groups would be 2.25 to 4.0 kg heavier than the control group (based on a 1.6 kg and 1.2 kg difference, respectively, between the plasma and PYE fed groups and the control group after the nursery phase).
Final weights at the end of the finishing phase are shown in Table 6. All pigs were removed from test on the same day for slaughter. Overall average daily gain during finishing for pigs from the PYE fed nursery group was higher (P<0.05) compared to gains of pigs from either of the other treatment groups. No significant differences for feed intake or feed efficiency were noted in the finishing phase.
Pigs fed plasma were numerically the heaviest coming out of the nursery phase, but numerically the lightest at the end of the finishing phase. A decrease in growth performance is commonly observed following plasma removal from the diet during the first period after the nursery phase, but no known work is available on the long term effects of feeding plasma during the nursery phase on finishing performance.
Animals previously fed plasma in the nursery would be expected to experience a brief performance dip, adjust to new diets, and finish with no further problems. As expected, growth rate during the first grower phase after the nursery phase was poorer (P<0.05) for the plasma-fed pigs in the nursery compared to the other two treatments. However, growth rate for the pigs fed plasma in the nursery was also poorer for the entire growing and finishing trial compared to either of the other treatments (P<0.05).
Table 5. Effect of feeding plasma or peptide (PYE) proteins with the addition of Carbadox on intestinal morphology of the nursery pig at 28 days.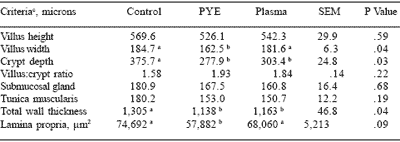
a,b Means in the same row lacking a common superscript differ significantly.
cLeast square means are reported from 8 pigs per treatment.
Carlson et al., 2001.
Table 6. Effect of feeding plasma or peptide (PYE) proteins without a feed grade antibiotic on finishing pig growth performance.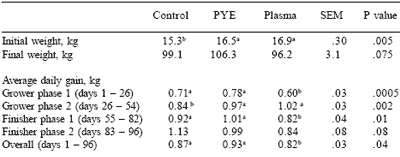
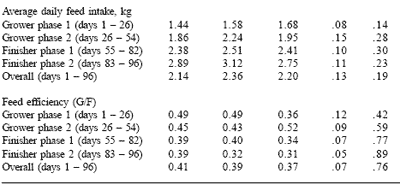
a,bMeans in the same row lacking a common superscript differ (P<0.05).
c Least square means are reported from 14 pigs per treatment.
With the exception of the second grower period (Table 6), pigs fed plasma in the nursery grew poorer in all finishing phases than pigs from either of the other treatments, suggesting a possible negative influence from plasma feeding for four weeks in the nursery. Pigs from the nursery control group might have been expected to finish 4 kg lighter than pigs from the plasma-fed nursery group, however, pigs fed the control treatment in the nursery overcame a significant weight deficit (1.6 kg) at the end of the nursery phase to finish 2.9 kg heavier than pigs fed plasma.
Pigs fed PYE in the nursery phase were expected to finish at approximately the same final weight as those from the plasma fed group. However, pigs fed PYE in the nursery finished 10 kg heavier than pigs fed plasma in the nursery, even though the weights of the two groups after the nursery trial were not significantly different. Again, there is the implication that either plasma inclusion in nursery diets under these conditions had a negative impact on post-nursery growth rate, or that PYE feeding in the nursery promoted a positive effect on finishing growth rate.
The hypothesis that PYE had a positive influence on post-nursery performance is substantiated in part by the comparison of post-weaning growth rate of pigs fed PYE in the nursery versus that of pigs fed the control diets. According to the rule of thumb above, that is, that each additional unit of nursery gain should produce an additional 2 to 3 units of finishing gain, pigs from the PYE treatment group having a 1.2 kg advantage after the nursery phase compared to control fed pigs should have gained an additional approximately 2.5 kg (for a 3.5 kg total difference) advantage after the finishing period.
Instead, pigs fed PYE in the nursery were 7.2 kg heavier after the finishing phase than pigs from the control groups, approximately twice the expected difference. This effect could have a very large impact on throughput, considered the most critical factor in profitability once pigs are weaned and on feed.
Carcass data for pigs from the Missouri trials is recorded in Table 7. Increased growth rate may result in a fatter pig or a lower percent lean carcass; however, no significant differences were noted for adjusted 10th rib backfat, adjusted loin eye area or percent carcass lean between treatments.
AQUACULTURE
Potential uses of yeast extract nucleotides in combating the devastating losses incurred in shrimp production has been reviewed by Devresse (2000) and Dominy (2000). Yeast extract nucleotides appear to offer promise in supporting resistance to viral infections prevalent in this industry. While nucleotides have not been thought to be essential dietary (or that incidental dietary levels are adequate) because they are produced endogenously by most species, it now seems that nucleotides are needed in diets nutrients for mammals, fish and poultry. Further, these compounds may become critical when the animal is stressed by either disease or injury, and important during infancy and periods of rapid growth (Uauy, 1989). Mendoza et al. (2001) demonstrated that even a low level of added yeast extract nucleotides could promote weight gain, survival rate and efficiency of feed conversion in shrimp exposed to the white spot virus.
HUMAN NUTRITION APPLICATIONS: INFANT FORMULA
Several authors have noted the potential impact of nucleotides on immunity. Investigation with animals demonstrated that dietary nucleotide supplementation influenced immune function (Carver et al., 1991; Carver, 1994). Infants fed breast milk or nucleotide-supplemented infant formula exhibited increased natural killer cell activity compared with infants fed unsupplemented formula (Carver, 1994). Interleukin-2 production by stimulated mononuclear cells was higher in the nucleotide-supplemented group compared to standard SMA formula with no nucleotide supplementation (Carver et al., 1991). Pickering et al. (1998) suggested that dietary factors such as nucleotides play a role in the antibody response of infants to immunization. Infant formula fortified with nucleotides enhanced H. influenzae type b and diphtheria humoral antibody responses. He also noted that nucleotides are one of the components of human milk that have been identified as affecting immune function.
Table 7. Effect of feeding plasma or peptide (PYE) proteins without a feed grade antibiotic on carcass characteristicsa.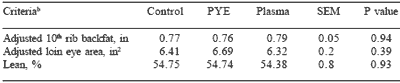
aadjusted to 113.6 kg BW
bLeast square means are reported from 14 pigs per treatment.
Thorell et al. (1996) addressed the potential of nucleotide supplementation in infant formulas. Interest has focused on the fact that human milk contains considerable amounts of nucleotides. The content of bovine milk is lower in nucleotides; and because bovine milk is the main ingredient for most infant formulas, nucleotides have been included in many formulas. They demonstrated that a small intestine homogenate would generate a net increase in pyrimidines and purines when incubated with milk containing nucleotides, whereas when incubated with infant formula devoid of nucleic acids, it did not.
Other studies provide additional insight as to how nucleotide supplementation may benefit health. Carver (1994) suggested that an exogenous source of nucleotides via the diet may optimize the function of rapidly growing tissues. Improved nitrogen metabolism has also been demonstrated (Carver et al., 1991). Scopesi et al. (1999) noted an increase in erythrocyte dyphosphoglycerate concentration by supplementing neonatal rats with dietary nucleotides. This was hypothesized to release more oxygen to peripheral tissues, thereby providing positive effects on neonatal pathological conditions such as respiratory disease.
A ROLE IN DIETS FOR YOUNG ANIMALS: ENTERIC DISEASE
The influence of nucleotide supplementation for human infants on diarrhea illnesses also poses interesting potential for feeding livestock. Two of the study sites in the extended investigation of nucleotide-supplemented formulas by Pickering et al. (1998) collected diarrhea data for the 12-month duration of the trial. Observations were blinded with respect to nucleotide supplementation in the formula groups. The results showed that the ingestion of nucleotide-fortified formula or human milk was associated with a significant decrease in the incidence of diarrhea when compared with the incidence in the control formula group without nucleotides.
Results from a field trial (Spring, 2001) evaluating the effect of NuPro on performance and health (diarrhea) of weaned piglets give some insight into the potential of yeast extract nucleotides to reduce diarrhea in farm livestock. The study was conducted on a farm experiencing ongoing problems with E. coli. Sixty-four mixed sex pigs were divided to two dietary treatments, a standard control diet and the same diet with 4% NuPro substituted for potato protein.
Pigs starting the test at an approximate weight of 10 kg received 20 kg of the treatment diets. Average daily gain (466 and 442 g, respectively) and feed intake (810 and 803 g, respectively) were improved in the group given NuPro. Feed conversion ratios were improved (P<0.05) in the pigs fed diets containing NuPro compared to controls (1.7 vs. 1.8). A sharp reduction in the occurrence of diarrhea was noted in the NuPro group. In the control group, 28 of 32 pigs received multiple (2 to 4) diarrhea treatments. In the NuPro group, 5 of 32 pigs were treated with a single injection for diarrhea. The economic implications from this commercial trial are that a potential reduction of 12-fold or greater in diarrhea treatments may be possible with a yeast extract protein source.
NuPro was tested (Maribo, 2001) for weanling pigs as an alternative, easily digestible protein source for pigs by the cooperative research group of the Danish Bacon and Meat Council. The loss of meat and bone meal and blood plasma, with the potential of losing fish meal as well, has created a need for alternative protein sources. A total of 545 pigs were divided into 33 replicates per treatment. The control diet was formulated with the primary ingredients of wheat, barley, soy protein, fishmeal and whey.
NuPro replaced primarily fish meal and whey in the other treatment diet sequence. Pigs were fed test diets from weeks 2 to 4 of age before weaning, and in two phases from weeks 4 to 7 and weeks 7 to 12 of age post-weaning. NuPro was included at 2.5%. Pigs were weaned at approximately 4 weeks of age and data for average daily gain, feed intake, feed efficiency, disease treatments and mortality was recorded.
With 2.5% NuPro in the treatment diets, no significant difference was seen in frequency of disease treatments. An average of 2.4 days of treatment was recorded for all pigs, with 2.0 of those days being for diarrhea treatments. However, mortality differed (P<0.01) between treatment groups. Mortality was 4.8% in the control group compared to 1.4% in the NuPro group. Average daily gain and feed intake were improved (P<0.01) for the 8-week nursery trial. An index value used as a standard of comparison by this group was calculated from the test gains, feed production units and cost of both of these changes in units, was also calculated for this experiment. A Danish Index value of 105 was recorded for the NuPro groups compared to an Index value of 100 for the control groups (P=0.08).
Conclusions
In summary, NuPro as a source of dietary nucleotides offers great promise for the livestock feeding industry. Human, small animal and farm animal research has demonstrated the potential for improvements in performance and health. In the early stages of production research with NuPro, improvements in growth, intake and efficiency of feed utilization, improvements in intestinal morphology, and improvements in short and long term health have been demonstrated under a variety of conditions. It may be concluded that not only does NuPro have the potential to replace many sources of animal proteins for food animals; it also has the potential to benefit intestinal health and immune function.
References
Boren, C.A., M.S. Carlson, T.L. Veum, J.R. Turk and G.W. Tibbetts. 2001. A comparison between feeding plasma and peptide proteins on nursery pig growth performance and intestinal health. J. Anim. Sci. 79(Suppl. 1):41 (Abstr.).
Carlson, M.S., T.L. Veum, J.R. Turk, D.W. Bollinger and G.W. Tibbetts. 2001. A comparison between feeding either peptide or plasma proteins with or without a feed grade antibiotic on pig growth performance and intestinal health. Unpublished. University of Missouri.
Carver, J.D. 1994. Dietary nucleotides: cellular immune, intestinal and hepatic system effects. J. Nutr. 124(Suppl. 1):S144-148.
Carver, J.D. and W. A. Walker. 1995. The role of nucleotides in human nutrition. J. Nutr. Biochem. 6:58-72.
Carver, J.D., B. Pimentel, W.I. Cox and L.A. Barness. 1991. Dietary nucleotide effects upon immune function in infants. Pediatrics 88 (2):359. Devresse, B. 2000. Nucleotides – a key nutrient for shrimp immune system. Feed Mix Vol. 8, No. 3.
Dominy, S. 2000. Shrimp: Feeding for disease resistance. Feed International. February.
Mahan, D.C. 1999. Comparison of plasma protein and Ultimate Protein in the diets of starter pigs. Report to Alltech. The Ohio State University.
Maribo, H. 2001. Commercial products for weaners: NuPro 2000 as an alternative protein source for weaners. Report no. 256, The National Committee for Pig Production, Danish Bacon and Meat Council, Danske Slagterier, Denmark.
Mendoza, S., A. Romo-Leroux, A. Solis, A. Fisher. 2001. Effect of NuPro on shrimp performance in Ecuador. Report to Alltech. Ecuador. Pickering, L.K., D.M. Granoff, J.R. Erickson, M.L.
Masori, C.T. Cordle, J.P. Schaller, T.R. Winship, C.L. Paule and M. D. Hilty. 1998. Pediatrics 101 (2):242.
Power, R. and R. Murphy. Biologically active peptides: sources, production and nutritional importance. In Biotechnology in the Feed Industry. Proceedings of Alltech’s 15th Annual Symposium, (T.P. Lyons and K.A. Jacques, eds.) Nottingham University Press, Nottingham. p. 435- 447.
Scopesi, F., C.M. Verkeste, D. Paola, D. Gazzolo, M.A. Pronzato, P.L. Bruschettini and U.M.
Marinari. 1999. Dietary nucleotide supplementation raises erythrocyte 2.3-diphosphoglycerate concentration in neonatal rats. J. Nutr. 129:662- 665.
Spring, Peter. 2001. Effect of NuPro2000 on commercial piglet performance in Switzerland. Report to Alltech. Zurich, Switzerland.
Touchette, K.J., R.L. Matteri, C.J. Dyer, J.A. Carroll and G.L. Allee. 1999. Effects of weaning diet on pig performance and intestinal morphology. J. Anim. Sci. 77(Suppl. 1):194 (Abstr.).
Tsujinaka, T., M. Kishibuchi, S. Iijima, M. Yano and M. Monden. 1999. Nucleotides and intestine. J. Parenteral and Enteral Nutr. Sep-Oct; 23(Suppl. 5):S74-77.
Thorell, L., L Sjoberg and O. Hernell. 1996. Nucleotides in human milk: sources and metabolism by the newborn infant. Pediatr. Res. 40(6):845-852.
Tibbetts, G.W. 2000. Biopeptides in post-weaning diets for pigs: results to date. In Biotechnology in the Feed Industry. Proceedings of Alltech’s 16th Annual Symposium, (T.P. Lyons and K.A. Jacques, eds.) Nottingham University Press, UK.
Uauy, R. 1989. Dietary nucleotides and requirements in early life. I: Textbook of Gastroenterology and Nutrition in Infancy, (E. Lebenthal, ed.), Raven Press, Ltd., New York, USA. p.265-280.
Author: G. WALTER TIBBETTS
Alltech Inc., Nicholasville, KY, USA
I would like to ask about the best way to get the pure nucleotides from yeast? I mean the best manufacture protocol?







.jpg&w=3840&q=75)